Abstract
Swerilactones H–K (1–4) as four unprecedented secoiridoid trimers represent a new type of natural product, which has attracted much interest of natural chemists due to their novel skeletons and promising bioactivity. In order to well understand their MS fragmentation behaviors, they were investigated by electrospray ionization ion-trap time-of-flight multistage product ion mass spectrometry (ESI-IT-TOF-MSn) for the first time. The protonated molecules ([M+H]+) of swerilactones J and K, and deprotonated molecules ([M−H]−) of swerilactones H, J and K were readily observed in the conventional single-stage mass spectra (MS); however only the [M+Cl]− ion for swerilactone I was obtained in negative mode. Based on the MSn study, the fragmentation pathways of swerilactones H and I in negative mode, and swerilactones J and K in both positive and negative modes were proposed. The neutral losses of H2O, CO, CO2 and C2H4O moieties are the particular elimination from the precursor ions due to the presence of hydroxyl, δ-lactone and 1-O-ethyl moieties in their structures, of which the retro-Diels–Alder cleavage was the most particular dissociation. The fragment ions at m/z 341 and 291 in negative mode can be considered as the diagnostic ions for secoiridoid trimers. This investigation will provide valuable information for their fast characterization from complicated natural mixtures and extensive understanding their structural architectures.
Similar content being viewed by others
1 Introduction
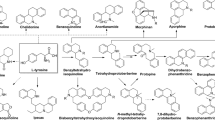
Natural products with diversities in chemical structures and pharmacological activities provide versatile candidates in drug discovery. Many natural chemists are committed to searching for novel compounds to enrich this library. Swerilactones H–K (1–4) (Fig. 1), unprecedented secoiridoid trimers from the traditional Chinese herb Swertia mileensis, represent a new type of natural product, which has attracted much interest of natural chemists due to their novel skeletons and promising bioactivity [1, 2]. However, this type of compound exists as minor components in plants, which presents challenges for their fast and reliable characterization [3]. Mass spectrometry (MS) with the associated high sensitivity and resolution well meets this requirement and has become the routine method in various aspects of medicinal chemistry [4–8]. Tandem MS techniques are particularly useful for ascertaining the relationship between precursor and product ions, by which the fragmentation rules and diagnostic ions of complicated compounds can be easily deduced [9–14]. The LCMS-IT-TOF mass spectrometer equipped with an electrospray ionization source linked to ion-trap and time-of-flight mass analyzers (ESI-IT-TOF) allows fast acquisition of multistage product ion spectra (MSn) with high accuracy and resolution in both positive and negative modes [15–17]. This feature leads to easier interpretation of the origin of product ions, which is suitable for investigating the structures of natural products. In this paper, we report for the first time a high-resolution MSn fragmentation study on swerilactones H–K (1–4) by ESI-IT-TOF mass spectrometer, which will provide valuable information not only for their fast characterization from complicated natural mixtures but also for a better understanding of their structural architectures.
2 Experimental
2.1 Apparatus and Analytical Conditions
MSn analyses were acquired on the LCMS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan). The mass resolution was about 10000 full width at half maximum (FWHM). Accurate masses were corrected by calibration using sodium trifloroacetate (CF3CO2Na) clusters. MS experiments were achieved in automatic pattern, and MSn experiments were performed in direct mode. Unless specified otherwise, analytical conditions were as follows: spray voltage, 4.50 and −3.50 kV; detector voltage, 1.60 kV; drying gas pressure, 100.0 kPa; nebulizing gas (N2) flow, 0.5 L/min; curved desolvation line (CDL) temperature, 200.0 °C; heat block temperature, 200.0 °C; equipment temperature, 40.0 °C; ion accumulation time, 10 ms; precursor ion selected width, m/z ± 3.0 Da, and selected time, 20 ms; collision induced dissociation (CID) collision time, 30 ms; collision energy, 50%; collision gas, 50%; and q = 0.251; scan range, m/z 100–1000 for MS. The Shimadzu Composition Formula Predictor was used to determine the molecular formula.
2.2 Chemicals and Samples
HPLC grade acetonitrile (CH3CN) was purchased from Merck (Merck Co. Ltd., Germany). HPLC grade formic acid was purchased from Aladdin (Aladdin Chemistry Co. Ltd. China). Deionized water was purified using a MingChe™-D 24UV Merck Millipore system (Merck Millipore, Shanghai, China).
Swerilactones H–K (1–4) were isolated from S. mileensis in our previous investigation, whose structures were unambiguously determined by extensive spectroscopic data and X-ray analyses [1]. Sample solutions were prepared by dissolving each sample in a solution of 85% CH3CN/H2O containing 0.05% formic acid to a final concentration of 0.2 mg/mL. The samples were introduced into the source via a syringe pump at a flow rate of 2 μL/min.
3 Results and Discussion
Before MSn investigation, the full-scan MS of compounds 1–4 in both positive and negative ion modes were acquired in automatic pattern. The protonated molecule ([M+H]+) and deprotonated molecule ([M–H]−) ions for swerilactones J (3) and K (4) were readily detected. However, swerilactones H (1) and I (2) only displayed [M–H]− or [M+Cl]− ion in negative mode. Therefore, the subsequent MSn study for swerilactones H and I (1 and 2) in negative mode, and for swerilactones J and K (3 and 4) in both positive and negative modes was performed, from which their fragmentation pathways were proposed (Figs. 2, 3, 4, 5). It should be noted that alternative ways of fragmentation that can reasonably interpret the product ions are also possible in addition to the proposed pathway.
3.1 ESI-IT-TOF MSn Fragmentations of Swerilactone H (1) in Negative Mode
In the single-stage mass spectrum of swerilactone H (1), the deprotonated molecular [M–H]− ion at m/z 567.1871 (1a) was readily obtained, corresponding to the molecular formula C30H32O11. When [M–H]− (1a) was selected as the precursor ion to perform MS2 experiment, multiple product ions (1b–1k) were observed. Among them, the ions at m/z 549 (1b) and 535 (1c) were deduced to be generated by the neutral losses of H2O and CH4O from 1a due to the presence of vicinal hydroxyl and methoxy groups [18, 19]. The product ions at m/z 519 (1d) and 505 (1e) were assigned to be the elimination of CH2O and the retro-Diels–Alder (RDA) cleavage of C2H4O from 1b [20]. Similarly, the cleavage of ring H by losing one C3H6O2 fragment from 1e generated ion 1f at m/z 431, and the most abundant ion at m/z 363 (1g) could be explained by subsequent loss of a C4H4O molecule by an RDA-like process [21]. It is noteworthy that two abundant ions at m/z 341 (1h) and 297 (1j/1j′) were readily observed in the MS2 spectrum, of which the ions at m/z 297 were present as double peaks at m/z 297.0792 (1j) and 297.1105 (1j′), corresponding to the chemical composition of C17H13O5 and C18H17O4 (Fig. 2). The ion at m/z 341 (1h) could be well interpreted by the RDA cleavage of ring F to lose a C11H14O5 part [22, 23]. Thus, the elimination of 44 Da can be attributed to the losses of C2H4O and CO2 to yield product ions 1j (m/z 297.0792) and 1j′ (m/z 297.1105) [24]. Likewise, the ion 1j could further lose a molecule of CO2 to generate ion 1k (m/z 253). In the MS3 experiment from the precursor ion 1g (m/z 363), two product ions at m/z 319 (1i) as base peak and 275 (1l) were detected, which were proposed to be arisen from the sequential loss of two CO2 molecules.
3.2 ESI-IT-TOF MSn Fragmentations of Swerilactone I (2) in Negative Mode
Compared to swerilactones H, J and K, swerilactone I (2) was more unstable in this MSn study, and thus, gave rise to less MSn information, which might be due to the presence of aldehyde group in the structure. In the full-scan mass spectrum, swrilactone I (2) displayed neither [M+H]+ nor [M−H]− ions, but an ion at m/z 571.1383 (2a) was readily obtained in the negative ion mode. This ion was ascribed with the chemical composition of C29H28O10Cl ([M+Cl]−) based on its high accordance in both accuracy (0.7 mDa) and isotopic abundance (83.9%) with those of the theoretical values. However, the origin of Cl− ion was unclear, which was always encountered in negative ESIMS investigation [25, 26]. In addition to the [M+Cl]− ion, two fragments at m/z 341 (2b) and 297 (2c) were observed with high abundance. The ion 2b corresponding to the loss of a C10H11O4Cl motif (rings G and H) from ion 2a could be explained by the RDA ring-opening of ring F, and the ion 2c was proposed to be generated by a further RDA process leading to the loss of a C2H4O part from ion 2b (Fig. 3). The above deduction was confirmed by the MS2 experiment on 2a, which gave rise to the expected ions 2b (m/z 341) and 2c (m/z 297), and the subsequent MS3 experiment on 2b in which the fragment ion at m/z 297 (2c) was further detected. Combined with the observation that the fragmentation ion 2c showed much higher abundance compared to the parent [M+Cl]− ion in the first stage mass spectrum, the following MS2 experiment was further performed on ion 2c (m/z 297) to generate three characteristic ions at m/z 253 (2d), 223 (2e) and 209 (2f). The ion 2d was attributed to the neutral loss of a CO2 moiety from ion 2c, and the ions 2e and 2f were corresponding to the elimination of one CH2O or CO2 parts from 2d. In the MS3 experiment on ion 2d, the expected fragment ions 2e (m/z 223) and 2f (m/z 209) were readily displayed, which was in accordance with the above deduction. When ion 2e was further selected for MS4 experiment, a fragment ion at m/z 195 (2g) corresponding to a 28 Da loss was obtained, which was deduced as the elimination of one CO moiety from the ion 2e [18].
3.3 ESI-IT-TOF MSn Fragmentations of Swerilactone J (3) in Positive and Negative Modes
In the positive full-scan mass spectrum, the [M+H]+ ion (3A) at m/z 537.1732 was readily detected, as well as the fragment ion (3B) at m/z 519 ([M+H–H2O]+) which was displayed as base peak in the subsequent MS2 experiment from 3A. The MS2 product ion at m/z 493 (3D) was designated as the RDA elimination of C2H4O moiety from 3A due to the presence of 1-O-ethyl group in the structure (Fig. 4). Similarly, the ion 3F (m/z 475) was formed by losing a C2H4O part from 3B, and further gave rise to ions 3H (m/z 447) and 3J (m/z 403) via consecutive elimination of one CO and one CO2 molecule [20]. The loss of a C2H2O segment was characteristic, by which the fragments 3E (m/z 477), 3I (m/z 433) and 3L (m/z 361) were formed from their respective parent ions 3B, 3F and 3J. In the MS3 spectrum from 3B, the ion 3C (m/z 501) corresponding to the loss of a H2O molecule was observed. With the elimination of a C3H4O2 part, the ion 3K (m/z 387) was produced, and further generated ions 3M (m/z 343) and 3N (m/z 315) by successive losses of CO2 and CO molecules. The ion 3O (m/z 307) in the MS3 spectrum was correspondent to the elimination of C6H6O3 moiety from precursor 3I.
The MSn investigation on swerilactone J (3) in negative mode provided more valuable information than that in positive mode. The first-stage mass spectrum displayed the [M–H]− ion at m/z 535.1602, assigned to the molecular formula C29H28O10. It should be noted that two fragmentation ions at m/z 491 (3b) and m/z 341 (3j″) were readily obtained with high abundance in addition to the [M–H]− ion, assigned to the molecular formula C28H27O8 and C19H17O6, respectively. The ion 3b was explained by the neutral loss of CO2 from the precursor ion 3a, and further confirmed by MS2 analysis in which the ion at m/z 491 was obtained as base peak. The ion 3j″ (C19H17O6) was proposed to be derived from 3a by neutral loss of a C10H10O4 part, due to the RDA cleavage of ring F [20]. When ion 3b (m/z 491) was selected as the precursor ion to perform MS2 experiment, prolific fragment ions were obtained, from which their fragmentation rules were proposed as shown in Fig. 4. Due to the high abundance of ion 3j″ (m/z 341.1030, C19H17O6) in the first-stage mass spectrum, subsequent MS2–4 experiments were applied on ion 3j″, from which a parallel fragmentation pathway was recognized. The neutral loss of CO2 from 3j″ provided ion 3 m′ (m/z 297.1081), and further produced ions 3n (m/z 279) and 3o (m/z 253) through the elimination of H2O or C2H4O part. In the MS4 experiment from the precursor ion 3n (m/z 279), characteristic product ions at m/z 235, 220 and 193 were obtained, of which the ion at m/z 235 was consist of two closed peaks at m/z 235.1087 (C17H15O, 3p) and 235.0749 (C16H11O2, 3p′), attributed to the neutral losses of CO2 or C2H4O moiety from 3n.
3.4 ESI-IT-TOF MSn Fragmentations of Swerilactone K (4) in Positive and Negative Modes
Structurally, swerilactone K (4) with an aromatic ring is obviously different from swerilactones H–J (1–3). The first-stage mass spectrum in positive mode displayed [M+H]+ ion (4A) at m/z 519.1651, corresponding to the molecular formula C29H26O9. The subsequent MS2 experiment from 4A yielded two high-abundance ions 4B (m/z 475) and 4C (m/z 457, base peak), attributed to the successive losses of C2H4O and H2O parts, in combination with three minor ions at m/z 299 (4J), 281(4k) and 253 (4L). The ion 4J was interpreted by the neutral loss of C10H8O3 from the precursor 4B due to the RDA cleavage of ring F, and further gave rise to ions 4K (m/z 281) and 4L (m/z 253) by the elimination of a molecule of H2O and CO (Fig. 5). This deduction was also confirmed by the MS3 analysis from the parent ion 4B. When ion 4C (m/z 457) was applied for the MS4 experiment, the most intensive ion at m/z 439 (4D) was readily detected, ascribe to the loss of H2O, together with a series of fragment ions 4E−4I.
In the negative ion mode, sweilactone K (4) gives rise to the deprotonated ion at m/z 517.1511, correlated to the molecular formula C29H26O9. The following MS2 experiment on 4a provided versatile fragments with ion at m/z 473 (4d) as base peak which was further applied for MS3 spectrum. Based on the above experiments, the fragmentation rules for swerilactone K (4) in negative mode were concluded. The minor ions at m/z 499 (4b) and 489 (4c) in MS2 spectrum were derived from neutral loss of H2O and CO from the precursor 4a. The most abundant ion 4d (m/z 473) generated from 4a by the RDA elimination of C2H4O segment, can further give rise to ions at m/z 427 (4e), 383 (3f) and 339 (4h) by sequential losses of CH2O2, CO2 and CO2 parts. In the MS3 spectrum from 4d, the product ions 4j (m/z 268), 4k (m/z 267), 4m (m/z 239) and 4n (m/z 211) could be explained by the consecutive elimination of C11H9O4 radical, hydrogen radical, CO and CO, respectively.
4 Conclusion
The ESI multistage product ion mass spectra (MSn) of swerilactones H–K were obtained for the first time by LCMS-IT-TOF, from which their fragmentation pathways were deduced. This investigation suggested that these molecules were unstable in this MSn study, especially for swerilactone I. The losses of H2O, CO2, CO and C2H4O moieties were the particular elimination from the precursor ions due to the presence of hydroxyl, δ-lactone and 1-O-ethyl groups. In particular, the RDA dissociation was the most common fragmentation rule which might correspond to the fused six-membered rings in their structures. It is important to note that the loss of CO2 and C2H4O can be unambiguously distinguished by high-resolution mass spectrometry. Structurally, swerilactones H–K share a closely related skeleton with the main difference located at rings F, G and H. Therefore, the conservative moiety (rings A to E) leads to the common fragments at m/z 341 and 291 in negative mode, which can be considered as the diagnostic ions for secoiridoid trimers. The present MSn fragmentation study on swerilactones H–K (1–4) by ESI-IT-TOF mass spectrometer will provide valuable information not only for their fast characterization from complicated natural mixtures but also for a better understanding of their structural architectures.
References
C.A. Geng, L.J. Wang, X.M. Zhang, Y.B. Ma, X.Y. Huang, J. Luo, R.H. Guo, J. Zhou, Y. Shen, A.X. Zuo, Z.Y. Jiang, J.J. Chen, Chem. Eur. J. 17, 3893–3901 (2011)
R.A. Hill, A. Sutherland, Nat. Prod. Rep. 28, 1031–1034 (2011)
C.A. Geng, X.M. Zhang, Y.B. Ma, J. Luo, J.J. Chen, J. Nat. Prod. 74, 1822–1825 (2011)
G.Z. Xin, J.L. Zhou, L.W. Qi, P. Li, Comb. Chem. High Throughput Screen 14, 93–103 (2011)
L. Hoffer, J.P. Renaud, D. Horvath, Comb. Chem. High Throughput Screen 14, 500–520 (2011)
M. Zhou, H. Luo, Z. Li, F. Wu, C. Huang, Z. Ding, R. Li, Comb. Chem. High Throughput Screen 15, 306–315 (2012)
L. Tokes, G. Jones, C. Djerassi, J. Am. Chem. Soc. 90, 5465–5477 (1968)
G.C. Kearney, P.J. Gates, P.F. Leadlay, J. Staunton, R. Jones, Rapid Commun. Mass Spectrom. 13, 1650–1656 (1999)
Q.R. Li, G.Q. Yan, T.F. Ge, Rapid Commun. Mass Spectrom. 21, 2843–2852 (2007)
R. Li, Z.J. Wu, F. Zhang, L.S. Ding, Rapid Commun. Mass Spectrom. 20, 157–170 (2006)
Q.R. Li, G.Q. Yan, T.F. Ge, Rapid Commun. Mass Spectrom. 22, 373–378 (2008)
Á. Peralbo-Molina, F. Priego-Capote, M.D. Luque de Castro, J. Agric. Food Chem. 60, 11542–11550 (2012)
K. Tóth, L. Nagy, A. Mándi, Á. Kuki, M. Mézes, M. Zsuga, S. Kéki, Rapid Commun. Mass Spectrom. 27, 553–559 (2013)
K. Ablajan, A. Tuoheti, Rapid Commun. Mass Spectrom. 27, 451–460 (2013)
C.A. Geng, Y.B. Ma, X.M. Zhang, S.Y. Yao, D.Q. Xue, R.P. Zhang, J.J. Chen, J. Agric. Food Chem. 60, 8197–8202 (2012)
B. Wang, W.Y. Chu, Z. Li, J.Q. Hou, S.L. Huang, T.M. Ou, J.H. Tan, L.K. An, D. Li, L.Q. Gu, Z.S. Huang, Rapid Commun. Mass Spectrom. 24, 2781–2786 (2010)
S.I. Kawano, Rapid Commun. Mass Spectrom. 23, 907–914 (2009)
J. Bolleddula, W. Fitch, S.K. Vareed, M.G. Nair, Rapid Commun. Mass Spectrom. 26, 1277–1290 (2012)
E.K. Jeong, S.Y. Lee, S.M. Yu, N.H. Park, H.S. Lee, Y.H. Yim, G.S. Hwang, C. Cheong, J.H. Jung, J. Hong, Rapid Commun. Mass Spectrom. 26, 1661–1674 (2012)
J. Ye, J.J. Qin, J. Su, S. Lin, Y. Huang, H.Z. Jin, W.D. Zhang, Rapid Commun. Mass Spectrom. 27, 2159–2169 (2013)
P.C. Eklund, M.J. Backman, L.A. Kronberg, A.I. Smeds, R.E. Sjöholm, J. Mass Spectrom. 43, 97–107 (2008)
Y.X. Zhang, Q.Y. Li, L.L. Yan, Y. Shi, Rapid Commun. Mass Spectrom. 25, 2173–2186 (2011)
A.D. Lucas, J. Fernandez-Gadea, N. Martin, R. Martinez, C. Seoane, Rapid Commun. Mass Spectrom. 14, 1783–1786 (2000)
J.J.J. van der Hooft, J. Vervoort, R.J. Bino, J. Beekwilder, R.C.H. de Vos, Anal. Chem. 83, 409–416 (2011)
W. Ding, K. Liu, X.J. Liu, H.X. Luan, C.F. Lv, T. Yu, G.M. Qu, J. Appl. Polym. Sci. 129, 2057–2062 (2013)
E.M. Thurman, J. Mass Spectrom. 41, 1287–1297 (2006)
Acknowledgements
This study was financed by the Youth Innovation Promotion Association (CAS), the West Light Foundation of CAS (Western Youth Scholars “A”), the Hundred Talents Program of CAS, and the Program of Yunling Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Geng, CA., Chen, JJ. A Fragmentation Study on Four Unusual Secoiridoid Trimers, Swerilactones H–K, by Electrospray Tandem Mass Spectrometry. Nat. Prod. Bioprospect. 6, 297–303 (2016). https://doi.org/10.1007/s13659-016-0114-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13659-016-0114-6