Abstract
Key message
We tested the life span of fine roots of Chinese fir trees and understory plants in two stands in subtropical China. Fine roots from understory plants were much more ephemeral than those from trees. The life span of fine roots of understory plants and Chinese fir was shorter in the younger than in the older stand, although most of the factors affecting fine-root life spans were similar between trees and understory plants.
Context
Understory fine root can contribute significantly to total fine root biomass and belowground carbon.
Aims
The contribution of understory vegetation to belowground carbon and nutrient cycling is often neglected in forest stands. Potential differences in fine-root life span between understory and trees remain poorly known. This study aimed to document fine-root life spans in trees and understory plants in two Chinese fir plantations with different ages.
Methods
We measured fine-root (≤2 mm in diameter) life span for trees and understory vegetation in 16- and 88-year-old Chinese fir plantations in southern China during 4 years with minirhizotron. Factors controlling fine-root life spans were identified with Cox proportional hazards regression.
Results
Fine roots were more ephemeral in understory plants than in trees in the two plantations. Fine-root life spans for both trees and understory plants were longer in the older than in the younger plantation. Root diameter at appearance, rooting depth, and season of emergence had a significant effect on fine-root life span.
Conclusion
These results highlight the importance of taking into account understory fine-root life span estimates when assessing the dynamics of fine-root recycling in Chinese fir forests.
Similar content being viewed by others
1 Introduction
Annually, between 10 and 60 % of net primary productivity (NPP) in terrestrial ecosystems can be cycled through fine roots (≤2 mm in diameter) (Jackson et al. 1997; Silver and Miya 2001; Ruess et al. 2003). Therefore, fine-root turnover represents a major pathway of C and nutrient flow from plant to soil and is fundamental to both forest NPP and C sequestration (Strand et al. 2008). Root life span, or root turnover rate, determines how fast C is cycled through the root biomass pool, reliable estimates of which are required by many ecosystem models. Fine-root life span can range from a few days to more than a decade and varies among species and across sites (Eissenstat and Yanai 1997; Peek 2007; Guo et al. 2008a; Strand et al. 2008; McCormack et al. 2012). However, ecosystem models have incomplete understanding on this critical belowground process (Jackson et al. 2000; McCormack et al. 2012, 2013; Chapin et al. 2009; Iversen 2010; Chen et al. 2014). One major area of uncertainty in mixed-species forests is the contribution made to root turnover at the stand level by understory vegetation. This is the focus of this paper.
Many forests have substantial understory communities (mainly herbs and shrubs), which can allocate a greater percentage of their photosynthate belowground than do trees (Raich and Tufekciogul 2000). Depending on the calculation assumptions used, typical ratios of belowground to aboveground biomasses of vegetation in forests range from about 0.3 to 0.5, but for grasslands, the corresponding ratios are about five times larger (Robinson 2007). Although understory plants represent a relatively minor component of the whole biomass of forest ecosystems, their roots could contribute significantly to the total fine-root biomass and belowground C dynamics of a forest stand particularly if they had faster turnover times than the fine roots of trees (Bakker et al. 2006; Helmisaari et al. 2007; Achat et al. 2008; Børja et al. 2008). This possibility remains untested because most root studies in forest ecosystems have focused on tree fine roots, while understory fine roots are generally excluded from analyses or are not distinguished from those of trees (Yang et al. 2004; Helmisaari et al. 2007; McCormack et al. 2012). This omission could underestimate belowground C and nutrient fluxes derived from fine-root turnover data, especially in subtropical forests where the understory vegetation develops vigorously under the tree canopy. Chen et al. (2013) estimated that as much as 68 % of the total fine-root biomass was composed of fine roots of understory vegetation in an overmature Chinese fir (Cunninghamia lanceolata (Lamb) Hook.) stand in southern China. However, apart from knowledge of fine-root biomass, so far, we know little about how the fine-root life spans of understory plants differ from those of trees in the same forest stand. A global meta-analysis by Chen and Brassard (2012) showed that fine roots of trees generally have longer life span, followed by grasses, lianas, shrubs, and forbs. Since herbs and shrubs are generally the dominant components of understory vegetation in many forest ecosystems, it is reasonable to expect shorter fine-root life spans for understory than for the trees with which they grow. Separately, estimating fine-root life spans of both trees and understory is thus essential to accurately model stand fine-root turnover.
The relative amounts of fine roots of different growth forms (herbs, shrubs, trees) in a specific forest ecosystem may vary with soil nutrients, management regimes, or stand development (e.g., Børja et al. 2008 and Chen et al. 2013). The composition of understory vegetation itself may also change temporally and spatially, e.g., more shrubs and fewer herbs in older canopy open stands (Chen et al. 2013). Besides, fine-root distribution (Børja et al. 2008; Chang et al. 2012), morphology (Claus and George 2005; Rosenvald et al. 2011, 2013), and belowground carbon allocation (Smith and Resh 1999; Giardina and Ryan 2002; Ryan et al. 2004) of trees may change with stand development or across soil nutrient gradient. All these aspects imply that, even for a specific forest type, interstand variations in overall fine-root life span are expected. Assuming a fixed value for fine-root life span for a specific type of forest, as in many current ecosystem models (McCormack et al. 2015), is unrealistic.
Accurately modeling interstand fine-root life spans is contingent on the separate insight into factors affecting fine-root life spans of understory plants and trees. Fine-root life span is affected by several factors, both intrinsic (e.g., tree species, allocation of photosynthate, root diameter, root order, season of emergence, rooting depth, mycorrhizal colonization) and extrinsic (including soil temperature, soil nitrogen (N) and water availability, soil biota, rhizosphere microorganisms, and atmospheric CO2 concentration) (Peek 2007; Chen and Brassard 2012; Eissenstat et al. 2013). For example, fine-root life span is lengthened with increases in root diameter, root order, and rooting depth (Anderson et al. 2003; Baddeley and Watson 2005; Espeleta and Donovan 2002; Gu et al. 2011; Hansson et al. 2013). Despite this growing body of information, we still have a limited understanding about how differently fine-root life span is regulated between understory plants and trees.
Chinese fir is one of the most important plantation trees in southern China, with a planting history over 1000 years (Chen et al. 2005b). Its planting area has reached over 12 million ha, accounting for about 6.5 % of all plantation forests in the world (FAO 2006; West 2006). Regular harvesting and replanting have resulted in mosaics of Chinese fir plantations with various development stages. In the present study, two Chinese fir stands (16- and 88-year-old), with different dominance and composition of understory vegetation, were chosen in a small watershed of Northern Fujian, China (Chen et al. 2013). Minirhizotron tubes were installed in these two stands, and long-term data were collected to test the hypothesis that (i) understory vegetation has more ephemeral fine roots than trees under both stands and that (ii) there are between-stand differences in fine-root life spans both for understory plants and for trees.
2 Materials and methods
2.1 Site description
The experiment was conducted on a small watershed in Wangtai town (26° 28′ N, 117° 57′ E, 200 m a.s.l.), Nanping city, Fujian province, China. The climate is of the subtropical monsoon type, with a mean annual air temperature of 19.3 °C. Mean annual relative humidity is 83 %. Mean annual precipitation is 1669 mm, which occurs mainly from March to August. According to Chinese soil classification, the soil is a red soil (State Soil Survey Service of China 1998), equivalent to hapludult in USDA Soil Taxonomy (Soil Survey Staff of USDA 1999). The soil is acidic and developed on deposit derived from deeply weathering products of Cretaceous Period granite and conglomerate. The soil profile is well developed and characterized by a B horizon of reddish- or yellowish-brown clay due to the accumulation of iron oxides. The dominant vegetation of the region was once evergreen broad-leaved forests, which were consecutively felled and planted with Chinese fir. This region is now a mosaic of different aged Chinese fir plantations.
The 16- (middle-aged, active growth) and 88-year-old (overmature, growth decline) Chinese fir stands were selected to represent two contrasting growth stages (Table 1), which corresponds to the stem exclusion stage and the understory reinitiation stage of stand development, respectively (Oliver and Larson 1996). These stands were carefully selected so that they differed only slightly in soil nutrients but contrasted in understory vegetation abundance and composition (Chen et al. 2013). The understory vegetation in the 16-year-old stand has a ground coverage of 90 %, composed mainly of herbs (such as Dryopteris austrtiaca, Woodwardia japonica, and Angiopteris fokiensis) and sparse shrubs (such as Maesa japonica, Ardisia punctata, and Paederia scandens). The 88-year-old stand has fully developed understory vegetation with 100 % ground coverage, characteristic of the understory reinitiation stage of stand development (Oliver and Larson 1996). The shrubs were dominated by M. japonica, Symplocos sumuntia, and Rubus buergeri and the herbs composed mainly of A. fokiensis and W. japonica.
2.2 Minirhizotron installation, image collection, and analyses
In June 2006, three 20-m × 20-m plots, separated from one another by at least 30 m, were established in each stand. In studies of tree fine-root dynamics, minirhizotron tubes are usually installed randomly in forest plots (Withington et al. 2006; Guo et al. 2008b) or near the bases of selected trees (Andersen et al. 2008; Krasowski et al. 2010). Because tree fine-root density is usually concentrated close to tree stems (Chen et al. 2005a; Yanai et al. 2006) and fine-root biomass at our sites is significantly smaller for trees than for understory vegetation in the overmature stand due to low stem densities (Table 1) (Chen et al. 2013), minirhizotron tubes were installed 0.5 m from the selected trees in both stands to intercept enough tree fine roots for survival analysis.
In April 2007, six or seven trees of average size were selected at each plot. One minirhizotron PVC tube was installed at 0.5-m distance from each stem, totaling 20 tubes per stand. Each tube was 90 cm long and 5 cm diameter and was installed at an angle of 45° from the soil surface. The vertical depth of the underground portion of each tube was between 55 and 60 cm. The upper open end of each tube was capped with a custom-built rubber stopper, and the portion of the tube above the soil was wrapped with thick black tape and then painted white to prevent light penetration and minimize radiative heating.
To allow reequilibration following minirhizotron installation and to improve contact between the soil and the tube surface, a 6-month time lag between installation and observation was adopted. Minirhizotron images were collected between October 2007 and December 2010 at monthly intervals, using a digital camera with I-CAP version 4.01 software (Bartz Technology Corp, Carpinteria, CA, USA). The image size was 14 mm × 18 mm, and usually, 42 (39–43) frames were taken for each tube. The software WinRHIZO TRON MF 2005a (Regent Instruments, Quebec, Canada) was used to digitize the images. Diameter and location of each root segment ≤2-mm diameter were recorded. Root branch order, extremely difficult to assess reliably in minirhizotron images, was not recorded.
Dates when individual root segments first appeared, died, and disappeared were also recorded. Fine roots were considered dead when they became black or shriveled and produced no new roots in subsequent viewings (Wells and Eissenstat 2001). The times of emergence and death were fixed at the midpoint between successive censuses. The fine roots were discriminated between those of trees or understory based on root color and morphology (Figs. S1 and S2). All fine roots other than those of Chinese fir were grouped as understory fine roots.
2.3 Fine-root extraction and chemical analyses
In July 2007, ten soil cores (60 cm in depth) were collected randomly from each plot (30 per stand) using a steel corer (6.8-cm diameter, 1-m length). Soil cores were then stored at 4 °C until processed. Cores were washed with tap water to remove adhering soil and accompanying organic debris. Living fine roots were then picked out based on color, texture, and shape of the roots and then grouped into two diameter classes (0–1 and 1–2 mm) (Chen et al. 2013). Fine roots were further separated into those of Chinese fir trees and of understory vegetation (herbs and shrubs). All fine-root samples were oven-dried (70 °C), weighed, ground, and passed through a 0.149-mm sieve. Samples of tree roots of 0–1-mm diameter were used for chemical analyses. C and N concentrations were measured by direct combustion (Vario EL III CNHOS Analyzer; Elementar Analysensysteme, Hanau, Germany). A subsample of fine roots was digested with sulfuric acid/perchloric acid, and phosphorus (P) concentrations were determined colorimetrically on a UV-visible spectrophotometer, using the ammonium molybdate ascorbic acid method (Kuo 1996).
2.4 Data analysis
Only newly emerged roots were included in fine-root life span analyses, and roots emerged before the first observation were excluded. Fine roots living at the end of the monitoring period were treated as censored. The median and mean life spans of the fine roots were calculated using the Kaplan-Meier survival function (Kaplan and Meier 1958), a nonparametric method that uses the product of survival probability in each time point before the present to estimate the cumulative survival rate over time. The differences in the survival probabilities of different groups (i.e., growth form, stand) were compared by the log-rank test. To test the effects of covariates on fine-root life span, Cox proportional hazards regression analyses (Cox 1972) were run separately for tree and understory fine roots with data of both stands combined, with root diameter at appearance, rooting depth, year of emergence, season of emergence, and stand as covariates. The term “stand” here represents a combination of all factors that differed between the stands other than root diameter at appearance, rooting depth, year of emergence, and season of emergence. In a proportional hazards model, the hazard of mortality of an individual root at time t (h (t, X)) is defined as the product of a baseline hazard function that is unspecified (h0 (t)) and a linear function of m covariates which is exponentiated (exp(β 1X1 + β 2X2 + …β m X m )) (Allison 1995):
In the Cox model, the partial likelihood method (Cox 1972) is adopted to estimate the β coefficient and to generate a chi-square statistic to test the null hypothesis that each β is equal to zero (Wells and Eissenstat 2001). The sign of β indicates whether the increase in a covariate would lead to increasing (positive sign) or decreasing (negative sign) hazard. Exp(β) was defined as a “hazard ratio” (Wells and Eissenstat 2001), which can be interpreted as one-unit increase in the value of covariate X associated with a 100[exp(β) − 1] percentage change in the hazard of mortality, controlling for all other covariates (Wells et al. 2002).
Differences between stands in the frequency distribution of the number of both tree and understory fine roots classified by diameter at appearance, rooting depth, year of emergence, and season of emergence were tested using a χ 2 test. Differences in tree fine-root chemistry between stands were tested by the independent sample t test.
All statistical analyses were conducted in SPSS 19.0 (SPSS Inc, Chicago, IL, USA). Significance levels were set at P = 0.05.
3 Results
3.1 Fine-root survival and life span
In total, 435 tree fine roots and 502 understory fine roots were tracked in the 16-year-old stands during the experiment, compared with 538 tree fine roots and 491 understory fine roots in the 88-year-old stands. Understory fine roots generally had smaller percentages of censored data than tree fine roots, especially in the older stand (13 vs. 32 %, respectively, Table 2).
In both stands, understory fine roots had significantly lower survival and hence much shorter life spans than tree fine roots (Fig. 1, Table 2). The survival probability of understory fine roots in the younger stand was significantly lower than that in the older stands (Fig. 1), with the median life spans of 93 ± 3 vs. 122 ± 9 days (Table 2). Tree fine-root survival was significantly lower in the 16-year-old stand than in the 88-year-old stand (Fig. 1); the corresponding median life spans were 274 ± 12 vs. 399 ± 31 days, respectively (Table 2).
3.2 Factors influencing fine-root life span
For tree fine roots in both stands, root diameter at appearance, rooting depth, year of emergence, season of emergence, and stand had significant influences on fine-root life span (Table 3). Coarser or deeper roots tended to live longer. More specifically, a 1-mm increase in root diameter increased the fine-root life span by 24 % on average, and a 1-cm increase in rooting depth prolonged fine-root life span by a more modest 4.1 %. Compared with tree fine roots that emerged in 2007, fine-root life span decreased by 53 % in 2009 roots and by 81 % in 2010 roots. Tree fine roots that emerged in winter lived longer than those emerging in spring. After accounting for the above covariates, stand difference still had a significant influence on tree fine-root life span. Compared with the 16-year-old stand, tree fine-root life span increased by 21 % in the 88-year-old stand (Table 3).
For understory fine roots in both stands, season of emergence, rooting depth, and stand difference were the significant factors influencing fine-root life span, while diameter at the time of appearance and year of emergence had no effect (Table 4). Season of emergence had the strongest effect on fine-root life span. Compared with those emerging in spring, fine roots emerging in summer and autumn decreased their life spans by 30 and 47 %, respectively. Fine-root life span apparently increased significantly with rooting depth, amounting, on average, to a 2.1 % increase in fine-root life span for every 1-cm increase in rooting depth. Stand difference was also a significant factor, even after accounting for the above covariates. Compared with the 16-year-old stand, understory fine-root life span increased by 17 % in the 88-year-old stand (Table 4).
By analyzing how factors influencing fine-root life span of different growth form differed between the younger and older stands (Figs. 2 and 3), possible mechanisms for the interstand variations in fine-root life spans for a specific forest type were suggested (Fig. 4).
Frequency of tree roots among a diameter at time of appearance, b rooting depth, c year of emergence, and d season of emergence in the 16- and 88-year-old Chinese fir plantations in southern China. Median16 and Median88 denote, respectively, the median values for the 16- and 88-year-old stand. P values indicate the significance of differences in frequency distribution between the two stands tested by the χ 2 test
Frequency of understory roots among a diameter at time of appearance, b rooting depth, c year of emergence, and d season of emergence in the 16- and 88-year-old Chinese fir plantations in southern China. Median16 and Median88 denote, respectively, the median values for the 16- and 88-year-old stand. P values indicate the significance of differences in frequency distribution between the two stands tested by the χ 2 test
A diagram summarizing the pathways through which stand differences could affect the overall life span of fine roots in an ecosystem comprising trees and understory plants. The variables in the rectangles are factors influencing root life span of tree or understory species. The solid and broken arrows, respectively, indicate significant effects or no effect, with “+,” “−,” and “0,” respectively, denoting a prolonged, shortened, or minimal effect of a factor on root life span. These results are based on the comparisons between the 88- and the 16-year-old Chinese fir stand (the latter used as the reference)
Since understory vegetation had much shorter fine-root life spans than trees, any change in the abundances of different plant growths must exert an influence on stand fine-root life span (Fig. 4), whether or not fine-root life spans of trees or understory themselves changed. The increase in the proportion of understory fine roots in the older relative to the younger stand (Table 1) should therefore have a negative effect on stand fine-root life span (Fig. 4).
For tree fine roots, fine-root frequency distributions differed significantly between the two stands for all the main factors influencing fine-root life span, i.e., diameter at appearance, rooting depth, year of emergence, and season of emergence (Fig. 2). There were more roots ≤0.5 mm in diameter but fewer >0.5 mm in diameter in the 88-year-old stand than in the 16-year-old stand, with median diameters of 0.58 vs. 0.65 mm, respectively (Fig. 2a). This indicates that tree fine roots were generally thinner in the older stand, a trait usually associated with shorter life spans (Fig. 4). Similarly, compared with the younger stand, there were fewer tree fine roots shallower than 30-cm depth, but more that were deeper than 30 cm in the older stand (Fig. 2b). This reflects an overall deeper rooting pattern for tree fine roots in the older stand, an effect prolonging root life span (Fig. 4). Compared with the 16-year-old stand, more tree fine roots in the older stand emerged in the first year (e.g., 2007, Fig. 2c) and fewer in the following years (e.g., 2008, 2009, and 2010, Fig. 2c), an effect which should extend tree fine-root life span since tree fine roots emerging in the latter years had higher mortality risk (Table 3). It is likely that a higher proportion of tree fine roots emerging in winter would extend tree fine-root life spans in the older stand since fine roots emerging in winter lived longer than those emerging in spring (Table 3). The term “stand difference” in Table 3 encompasses between-stand differences in a combination of factors other than root diameter at appearance, rooting depth, year of emergence, and season of emergence. For example, there were significant stoichiometric differences in tree roots between stands (Table 5). Such differences might influence root nutrient demands and metabolic activity, possibly prolonging tree fine-root life span more in the older than in the younger stand (Fig. 4).
For understory fine roots, there was a significant between-stand difference in the fine-root frequency of root diameter at the time of emergence (Fig. 3a). However, understory fine-root diameter did not influence the life span of those roots. This is probably because of the relatively narrow diameter range (95 % confidence interval 0.10–0.13 and 0.15–0.16 mm, respectively, for the 16- and 88-year-old stands) (Fig. 3a) when compared with the diameter distributions of tree fine roots (Fig. 2a). The rooting depth of the 88-year-old stand was more superficial than the 16-year-old stand (Fig. 3b), a factor which should be associated with a decrease in understory fine-root life span in the older stand relative to the younger stand. Because year of emergence itself did not influence understory fine-root life span (Table 4), a significant between-stand difference in root frequency distribution for year of emergence (Fig. 3c) would not exert an effect on the between-stand difference in understory fine-root life span (Fig. 4). There was also significant between-stand difference in the root frequency for season of emergence (Fig. 3d). Since only understory fine roots emerging in summer and autumn lived significantly shorter than those in spring (Table 4), the similar between-stand fine-root frequency during summer and autumn (Fig. 3d) should imply a limited effect of between-stand difference in season of emergence on understory fine-root life span. As before, the term stand difference in Table 4 represents between-stand differences in many factors other than root diameter at the time of appearance: rooting depth, year of emergence, and season of emergence, which might extend understory fine-root life span in the older stand compared with the 16-year-old stand (Fig. 4).
In summary, even for a specific forest type, variations in overall stand fine-root life span could arise from altering the stand’s growth form composition (trees vs. understory species) and by changing fine-root life spans of both trees (e.g., changed growth status) and understory plants (e.g., shifted understory composition) (Fig. 4).
4 Discussion
4.1 Fine-root life spans for understory and trees
Our results showed that understory fine roots had much shorter life spans than tree fine roots, which support our first hypothesis. This result echoes the finding of Chen and Brassard (2012) who, in a meta-analysis, compared fine-root life spans among biomes based on rhizotron and minirhizotron observations. They found that the fine roots of trees had the longest life spans, followed by shrubs and forbs. Using sequential soil coring, Finér and Laine (1998) also found that fine-root turnover rate of the field layer was faster than that of overstory trees in three Scots pine stands in southern Finland. Using minirhizotrons, Hansson et al. (2013), however, observed similar fine-root life spans for understory vegetation (including mainly grasses, ericoid shrubs, forbs, and some undefined understory trees) and overstory trees within the same root diameter class in Scots pine, silver birch, and Norway spruce stands in SW Sweden. The explanation for this discrepancy might be that Hansson et al. (2013) controlled root diameter differences during their comparisons between overstory and understory fine roots, while our study did not. The overstory fine roots were much coarser, and deeper, than understory fine roots under both stands, which might be the main causes for their longer life spans.
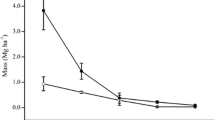
Since understory fine roots have shorter life spans than those of overstory trees, ignoring the contribution of understory fine roots will underestimate the total root turnover of a forest. In boreal and temperate forests, for example, tree fine roots contributed only 79–83 % of the forest’s total root turnover (Finér et al. 2011a, b). The remaining percentage was contributed by understory fine roots, and so, total root turnover could have been underestimated by 17–21 % by not including understory fine roots. The underestimation could be exaggerated in subtropical mature and overmature plantations because of their open tree canopies. Using estimates of fine-root biomass in Chen et al. (2013) and fine-root life spans obtained in the present study, fine-root mass turnover, calculated by dividing fine-root biomass by the median life span (in years), suggests that fine-root turnovers from understory vegetation and overstory trees were, respectively, about 98 vs. 212 g m−2 years−1 in the 16-year-old stand and 529 vs. 77 g m−2 years−1 in the 88-year-old stands. Total root turnovers would be underestimated by 32 and 87 %, respectively, in the younger and older stands, if understory fine roots are ignored.
Our results support the second hypothesis that there were significant between-stand differences in fine-root life spans both for trees and for understory even in a same forest type. Based on indirect methods using soil coring, two previous studies also reported interstand changes in fine-root turnover rate (root turnover rate is the reciprocal of root life span) for a same forest type. Helmisaari et al. (2002) found that fine-root turnover rate was fastest in the oldest stands of Scots pine (Pinus sylvestris L.) plantations of different ages (15-, 35-, and 100-year-old) in eastern Finland. Persson (1983) showed that fine-root turnover rates were 2.0 year−1 in a 20-year-old Scots pine stand and 0.7 years−1 in a 120-year-old stand. However, these studies did not discriminate fine roots of overstory trees and understory vegetation. Both ontogenic (e.g., tree age or tree physiology) and ecological factors (e.g., soil resources, competition) might result in interstand differences in tree fine-root life span. Using minirhizotron observations, Baddeley and Watson (2005) enabled comparison in root survivorship of Prunus avium trees of different ages at the same site in NE Scotland but found no significant tree age effect. Thus, the between-stand difference in tree fine-root life span in the present study might result from a combination of such factors, not merely tree age. The substantial changes in understory vegetation composition might be the most important factor resulting in the between-stand difference in understory fine-root life span. For practical reasons, we pooled the fine roots of all the sampled understory species into a single category “understory” due to the difficulty in distinguishing the roots of those species. Consequently, we were unable to attribute changes in understory fine-root dynamics to changes in understory species’ abundances in the community. Nevertheless, we suspect that changes in the abundances of understory species in the community, i.e., more shrubs and fewer herbs (mainly forbs), could explain the increased average understory fine-root life span in the older stand, because the fine roots of shrubs tend to be more perennial than those of herbs (Chen and Brassard 2012).
4.2 Factors influencing tree and understory fine-root life spans
The often-observed diameter effect on fine-root survivorship, i.e., prolonged root life span with increased root diameter (Eissenstat et al. 2000; Chen and Brassard 2012), was obvious for tree fine roots. Wells et al. (2002) reviewed possible explanations for shorter life spans of thinner roots, including greater vulnerability to environmental stresses such as parasitism, disease and drought, and higher specific construction costs and respiration rates. These factors may make the thinner roots more metabolically expensive to maintain in areas where soil nutrients are depleted. A lack of a diameter effect on understory fine roots could be due to their relatively narrow diameter range. Roots of understory species do not necessarily differ metabolically from those of trees, but it is simply more difficult to detect a diameter effect in understory vegetation because of their narrower diameter distributions.
As other studies have observed (Eissenstat et al. 2000; Guo et al. 2008b; Joslin et al. 2006), survivorship was greater with increasing soil depth both for tree and for understory fine roots. This could be related to smaller temporal fluctuations in temperature and water availability (Wells et al. 2002), reduced herbivory (Baddeley and Watson 2005), or downregulated maintenance respiration rates constrained by lower temperatures deeper in the soil (Eissenstat et al. 2000).
Tree fine-root life span decreased with the year of emergence, a finding similar to that of Guo et al. (2008b) who reported that life span decreased by 68 % for 2002 roots and by 110 % for 2003 roots compared with 2001 roots in a longleaf pine stand in Georgia, USA. In the first several months, disturbances during minirhizotron installation promoted production of pioneer roots, which can live much longer than fibrous roots (Zadworny and Eissenstat 2011). The relative proportion of pioneer roots in newly emerged roots would decrease and that of fibrous roots increases with time, which overall increases the mortality risk of the fine roots. However, in our study, the year of emergence did not affect understory fine-root life span, the precise cause of which remains to be explored.
Strong seasonality in fine-root survivorship is common in temperate forests (Eissenstat and Yanai 1997). So far, however, only one study has examined fine-root survivorship patterns in subtropical forest. In a subtropical longleaf pine forest, Guo et al. (2008b) detected that fine roots emerging in winter and spring generally lived longer than those emerging in summer and autumn. In the present study, when analyzing tree and understory fine roots separately, season of emergence influenced the fine-root life spans of trees and understory vegetation differently. We found longer tree fine-root life spans for the winter-emerging cohort than for the cohort emerging in spring. This might be because the cohorts emerging in winter (less favorable season) are less active and metabolize less nonstructural C (NSC) than those emerging in spring (López et al. 2001). The latter cohort is likely to be more active in absorbing available soil nutrients to support new aboveground growth during early parts of the season. We did not detect shorter tree fine-root life span for summer- and autumn-emerging cohorts, a phenomenon also found by Guo et al. (2008b). When grown in a mild subtropical monsoon climate, overstory trees might allocate enough current photosynthate belowground to support root functions even in the relatively warm summer and autumn season. By contrast, understory vegetation would face a strong seasonality in belowground C supply due to changing light availability and fluctuating photosynthetic rate, as well as limited NSC stores within the vegetation, before, during, and after canopy closure. Understory fine roots are thus prone to greater mortality during periods of carbohydrate shortage, e.g., in summer and autumn when maintenance respiration rates are high.
When controlling for other covariates (i.e., root diameter at the time of appearance, rooting depth, year of emergence, and season of emergence), stand difference still has a significant effect both on tree and on understory fine-root life span. For tree fine roots, this effect could be related to stoichiometric changes that reflect variations in their nutritional physiology with age and/or nutrient availability, as has been argued for terrestrial (Reich and Oleksyn 2007) and aquatic ecosystems (Helton et al. 2015). For understory fine roots, the reason for this effect of stand difference might be related to changes in root traits caused by shifting species’ composition within understory communities.
5 Conclusions
We showed that fine roots of understory plants were more ephemeral than those of the trees in both younger and older stands of Chinese fir. This highlights the significant contributions that understory vegetation could make to root turnover of the whole ecosystem in subtropical forests. Full understanding of belowground C and nutrient dynamics on forests requires that the separate contributions of fine roots of trees and understory plants be factored into ecosystem models when they are applied to multispecies communities. Of course, we acknowledge that the unreplicated nature of the stands, unavoidable in the forest chronosequence that we used, could potentially compromise observed between-stand differences in fine-root life spans. But, if such observations are generally applicable, our data suggest that fine-root life spans estimated for a single stand cannot necessarily be extrapolated reliably to other stands of different ages even if of the same forest type. It will be important for future work to quantify how fine-root life spans of different plant growth forms vary across a greater range of stands (e.g., stand development stages, soil nutrient gradient) within a specific forest type.
References
Achat DL, Bakker MR, Trichet P (2008) Rooting patterns and fine root biomass of Pinus pinaster assessed by trench wall and core methods. J For Res 13:165–175
Allison PD (1995) Survival analysis using the SAS system: a practical guide. SAS Institute Inc., Cary, NC, USA
Andersen CP, Phillips DL, Rygiewicz PT, Storm MJ (2008) Fine root growth and mortality in different-aged ponderosa pine stands. Can J For Res 38:1797–1806
Anderson LJ, Comas LH, Lakso AN, Eissenstat DM (2003) Multiple risk factors in root survivorship: a 4-year study in Concord grape. New Phytol 158:489–501
Baddeley JA, Watson CA (2005) Influences of root diameter, tree age, soil depth and season on fine root survivorship in Prunus avium. Plant Soil 276:15–22
Bakker MR, Augusto L, Achat DL (2006) Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 286:37–51
Børja I, De Wit HA, Steffenrem A, Majdi H (2008) Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol 28:773–784
Chang RY, Fu BJ, Liu GH, Yao XL, Wang S (2012) Effects of soil physicochemical properties and stand age on fine root biomass and vertical distribution of plantation forests in the Loess Plateau of China. Ecol Res 27:827–836
Chapin FS III, McFarland J, David McGuire A, Euskirchen ES, Ruess RW, Kielland K (2009) The changing global carbon cycle: linking plant–soil carbon dynamics to global consequences. J Ecol 97:840–850
Chen GS, Yang YS, He ZM, Xie JS, Gao R, Zeng HD (2005a) Effects of proximity of stems and tree diameters on fine root density in plantations. Acta Ecol Sin 25:1007–1011 (in Chinese)
Chen GS, Yang YS, Robinson D (2014) Allometric constraints on, and tradeoffs in, belowground carbon allocation and their control of soil respiration across global forest ecosystems. Glob Chang Biol 20:1674–1684
Chen GS, Yang YS, Xie JS, Guo JF, Gao R, Qian W (2005b) Conversion of a natural broad-leafed evergreen forest into pure plantation forests in a subtropical area: effects on carbon storage. Ann For Sci 62:659–668
Chen GS, Yang ZJ, Gao R, Xie JS, Guo JF, Huang ZQ, Yang YS (2013) Carbon storage in a chronosequence of Chinese fir plantations in southern China. For Ecol Manag 300:68–76
Chen HYH, Brassard BW (2012) Intrinsic and extrinsic controls of fine root life span. Crit Rev Plant Sci 32:151–161
Claus A, George E (2005) Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can J For Res 35:1617–1625
Cox DR (1972) Regression models and life tables. J R Stat Soc 34:187–220
Eissenstat DM, McCormack ML, Du Q (2013) Global change and root lifespan. In: Eshel A, Beeckman T (eds) Plant roots: the hidden half, 4th edn. Taylor and Francis Group/CRC Press, New York, pp 27–1–27–13
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Eissenstat DM, Yanai RD (1997) The ecology of root lifespan. Adv Ecol Res 27:1–60
Espeleta JF, Donovan LA (2002) Fine root demography and morphology in response to soil resources availability among xeric and mesic sandhill tree species. Funct Ecol 16:113–121
FAO (2006) Global forest resource assessment 2005. Food and Agricultural Organization of the United Nations, Rome
Finér L, Laine J (1998) Root dynamics at drained peatland sites of different fertility in southern Finland. Plant Soil 201:27–36
Finér L, Ohashi M, Noguchi K, Hirano Y (2011a) Factors causing variation in fine root biomass in forest ecosystems. For Ecol Manag 261:265–277
Finér L, Ohashi M, Noguchi K, Hirano Y (2011b) Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For Ecol Manag 262:2008–2023
Giardina CP, Ryan MG (2002) Total belowground carbon allocation in a fast-growing eucalyptus plantation estimated using a carbon balance approach. Ecosystems 5:487–499
Gu JC, Yu SQ, Sun Y, Wang ZQ, Guo DL (2011) Influence of root structure on root survivorship: an analysis of 18 tree species using a minirhizotron method. Ecol Res 26:755–762
Guo D, Li H, Mitchell RJ, Han W, Hendricks JJ, Fahey TJ, Hendrick RL (2008a) Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol 177:443–456
Guo DL, Mitchell RJ, Withington JM, Fan PP, Hendricks JJ (2008b) Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: root branch order predominates. J Ecol 96:737–745
Hansson K, Helmisaari HS, Sah SP, Lange H (2013) Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For Ecol Manag 309:58–65
Helmisaari HS, Derome J, Nöjd P, Kukkola M (2007) Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol 27:1493–1504
Helmisaari HS, Makkonen K, Kellomäki S, Valtonen E, Mälkönen E (2002) Below-and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. For Ecol Manag 165:317–326
Helton AM, Ardón M, Bernhardt ES (2015) Thermodynamic constraints on the utility of ecological stoichiometry for explaining global biogeochemical patterns. Ecol Lett 18:1049–1056
Iversen CM (2010) Digging deeper: fine root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci U S A 94:7362–7366
Jackson RB, Schenk HJ, Jobbágy EG, Canadell J, Colello GD, Dickinson RE, Field CB, Friedingstein P, Heimann M, Hibbard K, Kicklighter DW, Kleidon A, Neilson RP, Parton WJ, Sala OE, Sykes MT (2000) Belowground consequences of vegetation change and their treatment in models. Ecol Appl 10:470–483
Joslin JD, Gaudinski JB, Torn MS, Riley WJ, Hanson PJ (2006) Fine root turnover patterns and their relationship to root diameter and soil depth in a 14C-labeled hardwood forest. New Phytol 172:523–535
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Krasowski MJ, Lavigne MB, Olesinski J, Bernier PY (2010) Advantages of long-term measurement of fine root demographics with a minirhizotron at two balsam fir sites. Can J For Res 40:1128–1135
Kuo S (1996) Phosphorus methods of soil analysis. In: Sparks DL et al. (eds). Soil science society of America, Madison, Wisconsin, p 869–919
López B, Sabaté S, Gracia CA (2001) Fine‐root longevity of Quercus ilex. New Phytol 151(2):437–441
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195:823–831
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo DL, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppalammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere process. New Phytol 207:505–518
McCormack ML, Eissenstat DM, Prasad AM, Smithwick EA (2013) Regional scale patterns of fine root lifespan and turnover under current and future climate. Glob Chang Biol 19:1697–1708
Oliver CD, Larson BC (1996) Forest stand dynamics. Wiley, New York
Peek MS (2007) Explaining variation in fine root life span. Prog Bot 68:282–398
Persson HÅ (1983) The distribution and productivity of fine roots in boreal forests. Plant Soil 71:87–101
Raich JW, Tufekciogul A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90
Reich PB, Oleksyn J (2007) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci U S A 101:11001–11006
Robinson D (2007) Implications of a large global root biomass for carbon sink estimates and for soil carbon dynamics. Proc R Soc Lond B 274:2753–2759
Rosenvald K, Kuznetsova T, Ostonen I, Truu M, Truu J, Uri V, Lõhmus K (2011) Rhizosphere effect and fine-root morphological adaptations in a chronosequence of silver birch stands on reclaimed oil shale post-mining areas. Ecol Eng 37:1027–1034
Rosenvald K, Ostonen I, Uri V, Varik M, Tedersoo L, Lõhmus K (2013) Tree age effect on fine-root and leaf morphology in a silver birch forest chronosequence. Eur J For Res 132:219–230
Ruess RW, Hendrick RL, Burton AJ, Pregitzer KS, Sveinbjornssön B, Allen MF, Maurer GE (2003) Coupling fine root dynamics with ecosystem carbon cycling in black spruce forests of interior Alaska. Ecol Monogr 73:643–662
Ryan MG, Binkley D, Fownes JH, Giardina CP, Senock RS (2004) An experimental test of the causes of forest growth decline with stand age. Ecol Monogr 74:393–414
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Smith FW, Resh SC (1999) Age-related changes in production and below-ground carbon allocation in Pinus contorta forests. For Sci 45:333–341
Soil Survey Staff of USDA (1999) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. United States Department of Agriculture (USDA), Natural Resources Conservation Service, Washington DC
State Soil Survey Service of China (1998) China soil. China Agricultural Press, Beijing (in Chinese)
Strand AE, Pritchard SG, McCormack ML, Davis MA, Oren R (2008) Irreconcilable differences: fine-root life spans and soil carbon persistence. Science 319:456–458
Wells CE, Eissenstat DM (2001) Marked differences in survivorship among apple roots of different diameters. Ecology 82:882–892
Wells CE, Glenn DM, Eissenstat DM (2002) Changes in the risk of fine-root mortality with age: a case study in peach, Prunus persica (Rosaceae). Am J Bot 89:79–87
West PW (2006) Growing plantation forests. Springer, Berlin Heidelberg, Germany
Withington JM, Reich PB, Oleksyn J, Eissenstat DM (2006) Comparisons of structure and life span in roots and leaves among temperate trees. Ecol Monogr 76:381–397
Yanai RD, Park BB, Hamburg SP (2006) The vertical and horizontal distribution of roots in northern hardwood stands of varying age. Can J For Res 36:450–459
Yang YS, Chen GS, Lin P, Xie JS, Guo JF (2004) Fine root distribution, seasonal pattern and production in native and monoculture plantation forests in subtropical China. Ann For Sci 61:617–627
Zadworny M, Eissenstat DM (2011) Contrasting the morphology, anatomy and fungal colonization of new pioneer and fibrous roots. New Phytol 190:213–221
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31422012 and 31130013), the Natural Science Foundation of Fujian Province (2014J07005), and the National 973 Program (2014CB460602). We sincerely thank Yiding Yuan, Hua Ling, Zhengli Lu, Weiwei Wang, and Chaochao Huang for their help in field and laboratory work. We also thank the two anonymous reviewers whose comments improved the final version.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Ana Rincon
Contribution of co-authors
Guangshui Chen and Yusheng Yang designed the research.
Jinxue Huang and Guangshui Chen analyzed the data. Yusheng Yang, Zhijie Yang, Decheng Xiong, Jianfen Guo, Jinsheng Xie, and David Robinson contributed to data interpretation.
Jinxue Huang, Guangshui Chen, and David Robinson wrote the manuscript.
Rights and permissions
About this article
Cite this article
Huang, J., Chen, G., Yang, Z. et al. Understory fine roots are more ephemeral than those of trees in subtropical Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) stands. Annals of Forest Science 73, 657–667 (2016). https://doi.org/10.1007/s13595-016-0551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13595-016-0551-8