Abstract
• Context
Handroanthus impetiginosus is an ornamental tree used in urban forestry programs and landscaping, produces good timber, and has medicinal qualities. Clearing of natural forest has been increased and motivates to find techniques that optimize rhizogenesis to ex situ conservation and reforestation.
•Aims
This study evaluated Azospirillum brasilense as an enhancer of in vitro rooting of H. impetiginosus under different indole-3-butyric acid (IBA) pulse inductions and media formulations.
•Methods
In vitro shoots were induced with 0 to 50-μM IBA pulse for 3 days, transferred to two half-strength media (Murashige and Skoog salts with Gamborg’s vitamins [MSG] and woody plant medium), and inoculated or not with A. brasilense Cd or Az39 strains.
•Results
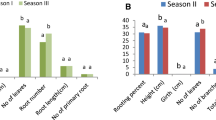
The statistic response surface methodology determined that both A. brasilense strains decreased auxin requirement for rooting up to 49 % in half-strength MSG. In this medium, Cd strain with 30-μM IBA pulse produced the 98 % of rooting shoots 21 days before uninoculated plants induced with the same IBA pulse. Also, the biometric parameter index increased from 127 to 286 % in shoots inoculated and induced with 0 to 30 μM IBA, relative to uninoculated.
•Conclusion
Our results show that biofertilization with A. brasilense significantly promotes in vitro rooting of H. impetiginosus decreasing auxin requirements and micropropagation costs.




Similar content being viewed by others
References
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 108:77–136. doi:10.1016/S0065-2113(10)08002-8
Bashan Y, Alcaraz-Melendez L, Toledo G (1992) Responses of soybean and cowpea root membranes to inoculation with Azospirillum brasilense. Symbiosis 13:217–228
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural and environmental advances (1997–2003). Can J Microbiol 50:521–577. doi:10.1139/W04-035
Carletti SM, Llorente BE, Rodríguez Cáceres EA, Tandecarz JS (1998) Jojoba inoculation with Azospirillum brasilense stimulates in vitro root formation. Plant Tissue Cult Biotechnol 4:165–174
Carley HE, Watson RD (1966) A new gravimetric method for estimating root-surface areas. Soil Sci 102:289–291
Cassán F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45:28–35. doi:10.1016/j.ejsobi.2008.08.005
Fibach-Paldi S, Burdman S, Okon Y (2012) Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol Lett 326:99–108. doi:10.1111/j.1574-6968.2011.02407.x
Gamborg OL, Miller R, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
George EF, Hall M, de Klerk G-J (2008) Plant propagation by tissue culture 3rd Edition. Volume 1. The background. Springer, The Netherlands, pp 65–173. doi:10.1007/978-1-4020-5005-3
González-Rodríguez J, Ramírez-Garduza F, Robert M, O’Connor-Sánchez A, Peña-Ramírez Y (2010) Adventitious shoot induction from adult tissues of the tropical timber tree yellow Ipé primavera (Tabebuia donnell-smithii; rose [bignoniaceae]). In Vitro Cell Dev Biol Plant 46:411–421. doi:10.1007/s11627-010-9304-9
Justiniano MJ, Fredericksen TS, Nash D (2000) Ecología y silvicultura de especies menos conocidas—Tajibos o lapachos (Tabebuia spp). Editora El País, Santa Cruz, Bolivia, p 60
Larraburu EE, Carletti SM, Rodríguez Cáceres EA, Llorente BE (2007) Micropropagation of photinia employing rhizobacteria to promote root development. Plant Cell Rep 26:711–717. doi:10.1007/s00299-006-0279-2
Larraburu EE, Apóstolo NM, Llorente BE (2010) Anatomy and morphology of photinia (Photinia × fraseri Dress) in vitro plants inoculated with rhizobacteria. Trees 24:635–642. doi:10.1007/s00468-010-0433-x
Larraburu EE, Apóstolo NM, Llorente BE (2012) In vitro propagation of pink lapacho: response surface methodology and factorial analysis for optimisation of medium components. Int J Forest Res 318258:9. doi:10.1155/2012/318258
Llorente BE, Apóstolo NM (1998) Effect of different growth regulators and genotype on in vitro propagation of jojoba. N Z J Crop Hortic Sci 26:55–62. doi:10.1080/01140671.1998.9514040
Llorente BE, Larraburu EE (2013) In vitro propagation of fraser photinia using Azospirillum-mediated root development. In: Lambardi M, Ozudogru A, Jain S (eds) Protocols for micropropagation of selected economically-important horticultural plants. Methods in molecular biology. Humana Press, New York, pp 245–258. doi:10.1007/978-1-62703-074-8_19
Lloyd GB, McCown BH (1980) Commercially-feasible micropropagation of Mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proc Int Plant Prop Soc 30:421–426
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assay with tobacco tissue cultures. Physiol Plant 15:473–497
Okon Y, Albrecht SL, Burris RH (1977) Methods for growing Spirillum lipoferum and for counting it in pure culture and in association with plants. Appl Environ Microbiol 33:85–88
Perrig D, Boiero M, Masciarelli O, Penna C, Ruiz O, Cassan F, Luna M (2007) Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol 75:1143–1150. doi:10.1007/s00253-007-0909-9
Pijut PM, Beasley RR, Lawson SS, Palla KJ, Stevens ME, Wang Y (2012) In vitro propagation of tropical hardwood tree species-a review (2001–2011). Propag Ornam Plants 12:25–51
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/. Accessed 10 Oct 2013
Rodríguez-Cáceres EA (1982) Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44:990–991
Russo A, Carrozza GP, Vettori L, Felici C, Cinelli F, Toffanin A (2012) Plant beneficial microbes and their application in plant biotechnology. In: Agbo EC (ed) Innovations in biotechnology., pp 57–72. doi:10.5772/31466, InTech, ISBN: 978-953-51-0096-6
SPSS Inc. (2003) SPSS for Windows, Version 12.0. Chicago, SPSS Inc.
Suarez IE, Jarma AJ, Avila M (2006) Development of an in vitro propagation protocol for roble (Tabebuia rosea Bertol DC). Temas Agrarios 11:52–62
Taiz L, Zeiger E (2010) Plant physiology, Fifth Edition, Sinauer Associates, 782 pages
Tetsumura T, Matsumoto Y, Sato M, Honsho C, Yamashita K, Komatsu H, Sugimoto Y, Kunitake H (2008) Evaluation of basal media for micropropagation of four highbush blueberry cultivars. Sci Hortic 119:72–74. doi:10.1016/j.scienta.2008.06.028
Vettori L, Russo A, Felici C, Fiaschi G, Morini S, Toffanin A (2010) Improving micropropagation: effect of Azospirillum brasilense Sp245 on acclimatization of rootstocks of fruit tree. J Plant Interact 5:249–259. doi:10.1080/17429145.2010.511280
Zhao D-Y, Tian Q-Y, Li L-H, Zhang W-H (2007) Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot 100:497–503. doi:10.1093/aob/mcm142
Funding
This research was supported by a grant from the Department of Basic Sciences, National University of Luján, Argentina. Ezequiel Larraburu is a doctoral student in Applied Sciences at the National University of Luján.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Douglass Jacobs
Contribution of the co-authors
Ezequiel Larraburu and Berta Llorente conceived and designed research and then analyzed the obtained data. Ezequiel Larraburu conducted experiments. Berta Llorente supervised the work and wrote the manuscript. Both authors read and approved the manuscript.
Rights and permissions
About this article
Cite this article
Larraburu, E.E., Llorente, B.E. Azospirillum brasilense enhances in vitro rhizogenesis of Handroanthus impetiginosus (pink lapacho) in different culture media. Annals of Forest Science 72, 219–229 (2015). https://doi.org/10.1007/s13595-014-0418-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13595-014-0418-9