Abstract
Climate change and global warming have worldwide adverse consequences. Biochar production and its use in agriculture can play a key role in climate change mitigation and help improve the quality and management of waste materials coming from agriculture and forestry. Biochar is a carbonaceous material obtained from thermal decomposition of residual biomass at relatively low temperature and under oxygen limited conditions (pyrolysis). Biochar is currently a subject of active research worldwide because it can constitute a viable option for sustainable agriculture due to its potential as a long-term sink for carbon in soil and benefits for crops. However, to date, the results of research studies on biochar effects on crop production show great variability, depending on the biochar type and experimental conditions. Therefore, it is important to identify the beneficial aspects of biochar addition to soil on crop yield in order to promote the adoption of this practice in agriculture. In this study, the effects of two types of biochar from agricultural wastes typical of Southern Spain: wheat straw and olive tree pruning, combined with different mineral fertilization levels on the growth and yield of wheat (Triticum durum L. cv. Vitron) were evaluated. Durum wheat was pot-grown for 2 months in a growth chamber on a soil collected from an agricultural field near Córdoba, Southern Spain. Soil properties and plant growth variables were studied in order to assess the agronomic efficiency of biochar. Our results show that biochar addition to a nutrient-poor, slightly acidic loamy sand soil had little effect on wheat yield in the absence of mineral fertilization. However, at the highest mineral fertilizer rate, addition of biochar led to about 20–30 % increase in grain yield compared with the use of the mineral fertilizer alone. Both biochars acted as a source of available P, which led to beneficial effects on crop production. In contrast, the addition of biochar resulted in decreases in available N and Mn. A maximum reduction in plant nutrient concentration of 25 and 80 % compared to nonbiochar-treated soils for N and Mn, respectively, was detected. This fact was related to the own nature of biochar: low available nitrogen content, high adsorption capacity, and low mineralization rate for N; and alkaline pH and high carbonate content for Mn. Our results indicate that biochar-based soil management strategies can enhance wheat production with the environmental benefits of global warming mitigation. This can contribute positively to the viability and benefits of agricultural production systems. However, the nutrient–biochar interactions should receive special attention due to the great variability in the properties of biochar-type materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Climate change and global warming have triggered worldwide efforts to identify problems and search for solutions in different productive sectors. The role of agriculture in mitigating climate change through options such as carbon sequestration in stable forms of soil organic matter is gaining interest worldwide (Stavi and Lal 2013). On the other hand, the reduction of CO2 emissions and the development of renewable energy sources in order to reduce energy dependence on fossil fuels are policy priorities in developed countries. This provides new opportunities for adding value to organic wastes through waste management processes such as pyrolysis: thermochemical decomposition in an atmosphere with low oxygen content. Among other products produced during pyrolysis which can be used as a source of renewable energy such as bio-oils and synthesis gas, a solid carbonaceous material (biochar) is obtained. The pyrolysis process stabilizes the carbon existing in the organic matter in a more resistant to chemical or biological decomposition, so that when incorporated into the soil is kept stable for longer and is not emitted to the atmosphere as would occur with the biomass decomposition. Therefore, biochar is attracting great interest for sustainable agriculture with potential benefits such as reduction in gas emissions, increase in soil carbon sequestration, and improvements in soil fertility and crop yield (Atkinson et al. 2010; Sohi et al. 2010; Stavi and Lal 2013).
The beneficial effects of biochar are determined primarily by some of its properties: high porosity, responsible for its high water retention capacity; high cation exchange capacity, which favors the retention of nutrients and prevent their loss; direct nutrient supply depending on the type of biochar; and the capacity of being a habitat for beneficial microorganisms, which can promote the release and uptake of nutrients by plants (Atkinson et al. 2010; Sohi et al. 2010). So, the chemical and biological stability of biochar, together with the aforementioned properties, have a high potential interest for agronomic systems in Mediterranean soils since they are generally poor in organic matter, which limits their fertility. In addition, the intensification of agricultural activities has accelerated processes such as degradation and loss of soil fertility, leading to increased production costs to maintain soil productivity. Therefore, strategies favoring preservation or increase of the soil organic matter content play a key role for maintaining crop productivity in agricultural systems (Pascual et al. 1999).
Biochar production as a waste management option can add value to several waste materials with great economic and environmental benefits. Spain has a high potential for generating biochar from agroforestry, livestock and agro-industrial wastes; however, the main limitation for implementation is its adequate use as quality amendments and fertilizers for agricultural use. At present, the results about the effects of biochar addition on crop production are inconclusive. They show either negative, zero, or positive responses depending on the type and properties of biochar as well as experimental conditions such as soil type, crop species, and environmental conditions. This makes necessary to further investigate biochar effects on crop production under specific site conditions (Jeffery et al. 2011). On the other hand, little information is available about the effects of biochar addition on key variables that cause differences in growth such as biomass allocation to leaves, stems, roots, and physiological variables, as also on the influence of biochar on the availability of nutrients, being the main novelty of this study.
Wheat is one of the most widely cultivated crops with a world production of about 650 million tons per year. Wheat is the third most-produced cereal after maize and rice (FAO 2012). Durum wheat has excellent food qualities such as high fiber content, minerals, and gluten. Spain is the sixth largest world producer of durum wheat with a 5 % of the total production and Andalusia (Southern Spain) is the leading producer of durum wheat in Spain (80 % of the national production) with a yearly production of one million tons. It represents an approximate value of 200 million Euros (BAMP 2012).
In the present study, durum wheat (Triticum durum) was grown under different treatments (biochar and mineral fertilization) in a controlled growth chamber. We used two types of biochar produced from wheat straw and olive tree pruning (Fig. 1). The objectives of this study were: (1) to evaluate the effects of the addition of two types of biochar on the growth and yield of durum wheat; (2) to compare different application rates of biochar; and (3) to study the effects of different levels of mineral fertilization in the presence and absence of biochar on wheat growth and yield.
a Olive tree pruning biochar, b wheat straw biochar, and c wheat plants in the growth chamber. The use of biochar obtained from biomass pyrolysis as a soil amendment is gaining interest to mitigate climate change and improve soil productivity. The potential benefits include a reduction in gas emissions, an increase in soil carbon sequestration and improvements in soil fertility and crop yield. These findings constitute a great incentive for the implementation of biochar-based strategies, which could contribute to the sustainability of agricultural systems
2 Materials and methods
2.1 Biochar characterization
Wheat straw and olive tree pruning were selected to produce the biochar samples as these are biomass waste materials commonly found in agricultural areas of Southern Spain. Biochar was produced by using a laboratory-scale pyrolysis equipment based on the Anila Stove design (Iliffe 2009). Biochar yield on a dry mass basis [100×(biochar mass/raw material biomass)] was determined and biochar samples were ground in a stainless steel mill to <2 mm before analysis and use in the plant growth experiment. The wheat straw showed a maximum pyrolysis temperature of about 370 °C, temperatures being higher than 300 °C for 1.6 h and the total process time being 4 h. The olive tree pruning presented a higher maximum pyrolysis temperature (about 450 °C), time with temperatures higher than 300 °C was ∼3 and total process time was ∼4 h. The biochar yield on a dry weight basis was clearly higher for the olive tree pruning (60 % compared to 38 %), which was favored by the presence of a considerable amount of soil impurities in the raw material.
Both biochar samples had an alkaline pH value close to 11. The olive tree pruning biochar showed higher liming (219 compared with 71 g kg−1, expressed as calcium carbonate equivalent) and bulk density values (0.66 compared with 0.19 g cm−3). The electrical conductivity (EC) value differed greatly among samples (1.5 dS m−1 for olive tree pruning biochar and 2.8 dS m−1 for wheat straw biochar) as also did the cation exchange capacity (CEC) values (21 and 47 cmol(+) kg−1 for olive tree pruning biochar and wheat straw biochar, respectively). The olive tree pruning had higher ash and N but lower total organic C content than the wheat straw biochar (391, 10, and 484 g kg−1, respectively, compared to 251, 8, and 628 g kg−1). Inorganic N (NO3 −–N + NH4 +–N) was not detected in any of the biochar samples. Olive tree pruning biochar had 0.8, 5.4, 86.0, and 2.0 g kg−1 of P, K, Ca, and Mg, respectively, against corresponding figures of 0.8, 8.0, 5.4, and 1.4 g kg−1 for wheat straw biochar. Both biochars showed germination indices (GI) above 60 and 90 % of the control for lettuce and cress, respectively, which are typical of nonphytotoxic materials.
2.2 Plant growth experiment
The soil used in this study was collected from the topsoil (0–20 cm) of a field adjacent to the Rabanales Campus (Universidad de Córdoba; 37° 56′ 04″N, 4° 43 ′05″W, Córdoba, Spain). The soil had a loamy sand texture (80 % sand, 14 % silt, and 6 % clay), pH (1:2.5 soil/water ratio) of 6.5 and electrical conductivity (1:5 soil/water ratio) of 0.06 dS m−1, 9.0 g kg−1 total organic carbon, 0.8 g kg−1 total nitrogen, 12.5 mg kg−1 Olsen-P, and 10.1 cmol(+) kg−1 cation exchange capacity.
The 2-month durum wheat (T. durum L. cv. Vitron) growth experiment began on January 25, 2012. Three levels of mineral fertilization were used: F0 (without mineral fertilization), F40 (“low”; mineral fertilization, 40 %) and F100 (“complete”; mineral fertilization, 100 %), providing a total of 0, 58, and 144 mL per pot during the cultivation cycle, respectively, of a full Hoagland nutrient solution. The mineral fertilization rates were defined based on previous experiments taking into account the amount of nutrient extracted by the wheat crop in 2 months (dry plant biomass produced and the nutrient sufficient ranges; Mengel and Kirkby 2001), and the number of plants per pot. The mineral fertilization was distributed over the growth period in weekly applications. Besides the nutrient solution, deionized water was applied on a daily basis to keep soil moisture near field capacity. Initially, the soil moisture was adjusted to achieve 80 % of the water retention capacity of soil and it was maintained during the growing season with daily watering. The weight of each pot was registered daily to measure crop evapotranspiration during the experiment. This variable was calculated as the sum of water loss per pot due to evaporation and plant transpiration.
For each biochar type, the design consisted in a factorial with 4 biochar application rates: 0, 0.5, 1, and 2.5 % w/w on a dry weight basis; and three levels of mineral fertilization, with five replicates per treatment. The required amount of biochar for each treatment was thoroughly mixed with soil. Plastic pots with a capacity of 500 mL (10 cm high and 7 × 7 cm) were filled with 350 g dry soil or soil–biochar mixture.
Initially and after biochar addition to soil, pH, EC, field capacity, and bulk density were determined in the biochar–soil mixtures. To estimate soil N and P availability in the pots, anionic and cationic exchange resin membranes (I-100 and I-200 types, Electropure Excellion, Laguna Hills, CA, USA) were used. Resin membranes were previously conditioned in the lab by immersing them in demineralized water at 82–90 °C for 48 h. After conditioning, 2 × 2 cm resin membranes were glued on a plastic holder to facilitate insertion into the soil. The membranes were positioned 5 cm below the soil surface.
The wheat seeds were placed in Petri dishes on a moistened paper in cold storage at 5 °C for 5 days before planting to favor seed germination. Subsequently, nine seeds were sown per pot and after germination (5 days) were removed to leave only six plants per pot, quantifying the number of germinated seeds in each pot.
Pots were randomly arranged in a growth chamber with a photoperiod of 16 h. Throughout the experiment, the average temperature of the growth chamber was 23 ± 3 °C, the relative humidity 42 ± 12 %, and the light intensity 250 μmol m−2 s−1 provided by Sylvania Cool White fluorescent tubes (F72T12/CW/VHO H298 160W). The position of the pots in the growth chamber was changed weekly to avoid the influence of microclimate variability.
The chlorophyll content of the youngest most fully expanded leaves was estimated from the SPAD value (SPAD 502 portable chlorophyll meter; Minolta Camera Co., Osaka, Japan), which was measured in the fourth, fifth, sixth, and seventh week of the experiment. After the last SPAD reading, and immediately before harvest, the youngest fully expanded leaf in each plant was cut, its surface area measured and its chlorophyll extracted with 96 wt % ethanol in order to calibrate SPAD values. Chlorophyll concentrations were determined according to Wintermans and de Mots (1965). In order to simplify, the chlorophyll data showed in the present study correspond to the seventh week measurement since all data showed a similar trend.
During the seventh week, the number of plants with ear was counted as an indicator of phenological stage (ear appearance), one plant per pot was harvested to evaluate the crop nutritional status, and five plants per treatment combination were randomly chosen to measure photosynthetic activity. We used an infrared gas analyzer (CIRAS-2, PP-System, Hitchin, UK). The measurements were performed at 380 ppm CO2 concentration, 200 cm3 min−1 air flow, and 25 °C leaf temperature. The leaves were introduced into the chamber and we took three measurements when gas exchange was stable. When the blade did not occupy the entire camera, a digital photograph was taken and the area measured with Image-Pro Plus 4.5 (Media Cybernetics, Bethesda, MD, USA), in order to correct the gas exchange measurements. The water use efficiency was calculated as the ratio between photosynthesis rate and stomatal conductance.
Plants were harvested after 2 months on March 26, 2012. At harvest, plant height and aboveground fresh weight were determined. Roots were separated from the soil and the resin membranes were air dried, and cleaned of soil particles. Plants were separated into leaves, stems, roots, and ear and obtained fresh weights. The leaves, ear, stem, and root were stored in individual paper bags and dried in an oven at 70 °C for at least a week, for subsequent determination of dry weight. Ear, grain, leaf, stem, and root mass fractions were calculated as the dry mass of ear, grain, leaf, stem, and root divided by total plant dry biomass, respectively.
2.3 Analytical methods
The following parameters were determined in the biochar samples: pH and EC in the 1:10 (w/v) biochar/water extract after stirring the mixture mechanically for 2 h. The pH was measured in the supernatant with a pH meter with electrode glass and the EC with a conductivity bridge, after centrifugation and filtering. The ash content was determined by a muffle furnace at 550 °C (TMECC 2002). The total organic carbon and total nitrogen were measured with an elemental analyzer (EuroVector, Milan, Italy). The biochar bulk density was estimated by weighing 10 mL of milled sample. The liming value of biochar was measured as calcium carbonate equivalency according to the 04.08-A method (TMECC 2002). Total P, K, Ca, and Mg were determined after dry ash sample digestion (04.12-C method; TMECC 2002). In the solution, P was determined colorimetrically (Murphy and Riley 1962), K by atomic emission spectroscopy, and Ca and Mg by atomic absorption spectrophotometry. CEC was measured by a modified ammonium–acetate compulsory displacement method (Gaskin et al. 2008). The GI was determined according to the method proposed by Zucconi et al. (1981) using cress (Lepidium sativum L.) and lettuce (Lactuca sativa L.).
In the soil, pH and EC were determined in a 1:2.5 and 1:5 (w/v) soil/water extract, respectively. Particle size distribution was determined by the pipette method and CaCO3 content was measured with a calcimeter. Field capacity was determined by placing soil in a 10 cm high cylindrical column, wetting it, letting it drain for 48 h, and then measuring the water content at a depth of 3–6 cm. The bulk density of the soil was determined by weighing 1000 mL of dry soil. The total organic carbon and total nitrogen were measured with an elemental analyzer (EuroVector, Milan, Italy). The soil available P was extracted with 0.5 M NaHCO3 (1:10, w/v) for 30 min and measured colorimetrically (“Olsen P”; Olsen and Sommers 1982). The CEC was measured by saturation with sodium at pH 8.5, which included washing with sodium acetate, ethanol, and, finally, 1 M ammonium acetate.
NH4 +, NO3 −, and PO4 3− were extracted from the resin membranes by shaking them in 50 mL of 2 M KCl for 1 h at 200 rpm in an orbital shaker. These extracts were used to calculate the amount of NH4 +–N and NO3 −–N by colorimetry, indophenol blue method, using a microplate reader (Sims et al. 1995). PO4 3−–P concentration in the extract was determined by the molybdenum blue method (Murphy and Riley 1962), and absorbance was measured with a microplate reader (D’Angelo et al. 2001).
The availability of nitrogen in labile organic forms was determined by analysis of dissolved organic nitrogen (DON) and N in soil microbial biomass. The former has proven to be an important source for plants in agricultural systems, whose availability goes unnoticed by the use of ion exchange resins. For this reason, fresh soil samples were extracted with 0.5 M K2SO4, passed through a 0.45 μm Millipore filter and total nitrogen determined in the extract using an automatic analyzer for liquid samples (TOC-V CSN+TNM-1 Analyzer, Shimadzu). DON was determined as the difference between the total and the inorganic (NO3 − and NH4 +) nitrogen content in the K2SO4 extract. Soil microbial biomass nitrogen (SMB-N) was also determined since it constitutes a source of N for plants in the short term, especially in soils with frequent drying–wetting cycles. SMB-N was obtained by the fumigation–extraction method (Vance et al. 1987) and determined with an automatic analyzer for liquid samples (TOC-V CSN+TNM-1 Analyzer, Shimadzu).
Aboveground plant material was dried at 70 °C for at least 72 h, weighed, digested in nitric/perchloric acid, and the resulting solution analyzed for Ca, Mg, Fe, Mn, Cu, and Zn by atomic absorption spectrophotometry; K and Na by flame emission; and P with the molybdenum blue color method of Murphy and Riley (1962). All physicochemical analyses were performed in duplicate and soil microbial biomass in triplicate. The results are presented on a dry weight basis (24 h at 105 °C).
2.4 Statistical analysis
Data were subjected to a two-way analysis of variance (ANOVA) with type III sums of squares, considering biochar application rate and mineral fertilization application rate as factors. Prior to ANOVA, data were square root-, arcsine-, or log-transformed to satisfy the normality and homoscedasticity assumptions. The program STATISTICA™ (version 8, StatSoft™) was used for statistical analysis.
3 Results and discussion
As explained before, the aim of this study was to evaluate the agronomic efficiency of two types of biochar produced from representative agricultural wastes of the Mediterranean area. Thus, main effects of biochar addition on soil properties and wheat physiology, growth, and yield were studied under different levels of mineral fertilization in a growth chamber experiment.
3.1 Effects of the mineral fertilization application
As expected, the increase in nutrient status of the soil due to mineral fertilizer addition resulted in an increase in wheat dry biomass variables: leaves, stem, root, and grain; regardless of the biochar used (Table 1). These results indicate that the nonfertilized soil had a nutrient limitation as expected due to its low cation exchange cation capacity, and clay and organic carbon contents. In addition, the biomass allocation changed with an increase in the mineral fertilization application rate, decreasing the percentage of leaf, stem, and root mass with respect to the total dry plant biomass but increasing both grain percentage and weight (Table 1, Fig. 2). The fertilizer application decreased the dry matter content of the aboveground part (Table 1). Furthermore, the addition of the mineral fertilization caused an increase in the total evapotranspiration, which must be related to the increase in plant biomass and hence crop evapotranspiration, as also in the chlorophyll content, revealing a better plant performance as mineral fertilization rate increased.
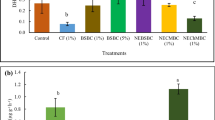
Effects of biochar application rate and mineral fertilization level on biomass production parameters (mean value ± standard error, n = 5; where absent, bars fall within symbols). F0 without mineral fertilization, F40 mineral fertilization 40 %, and F100 mineral fertilization 100 % (complete). Both biochars had little effect on wheat yield in the absence of the mineral fertilization. However, the combination of the mineral fertilization and biochar at the highest rate led to a significant increase in both grain percentage and biomass, indicating the beneficial effect of biochar addition to soil
In the case of wheat straw biochar addition, the mineral fertilization also increased the percentage of plants forming ears after the seventh week, suggesting that a higher nutrient content of soil accelerates plant phenology (Table 1). In addition, the mineral fertilization had a positive effect on gas exchange variables such as photosynthesis rate, stomatal conductance, and water use efficiency.
With respect to the aboveground nutrient concentration (data not shown), it decreased with increasing doses of mineral fertilizer due to a dilution effect since mineral fertilization application led to a greater dry matter plant production. When these results are expressed as nutrient extracted by the crop (Table 2), plant nutrient uptake increased after the mineral fertilization addition with a little effect on soil nutrient status at the end of the experiment. Only the nitrate concentration extracted by the resin increased with the mineral fertilization addition to soil. The poor nutrient status of the soil used in the present experiment and the mineral fertilization adjusted to crop demand could justify the above results. All these results are consistent with numerous studies on the effects of fertilization on growth and ecophysiology of plants (Gooding and Davies 1997; Hay and Walker 1989).
3.2 Effects of the biochar application
The beneficial effects of biochar addition on crop production may be determined by changes in soil characteristics and/or the availability of nutrients (Chan et al. 2007; Sohi et al. 2010). According to data prior to the implementation of the wheat crop, both biochars significantly increased soil pH from 6.5 in the control soil to 8.2 and 7.6 in the soil treated with the highest biochar application rate for olive tree pruning biochar and wheat straw biochar, respectively (P < 0.001). Biochar addition also increased the electrical conductivity of the 1:5 soil/water extract from 50 μS cm−1 in the control soil to 104 and 70 μS cm−1 in the soil treated with the highest biochar application rate for olive tree pruning biochar and wheat straw biochar, respectively (P < 0.001). In addition, wheat straw biochar addition determined a statistically significant increase in soil field capacity from 14.0 to 15.8 % (P < 0.05) and a decrease in soil bulk density from 1.56 to 1.49 g cm−3 (P < 0.001) at the highest biochar application rate. The effects on wheat seed germination in soil–biochar mixtures were not statistically significant for olive tree pruning biochar and decreased at the highest application rate of wheat straw biochar (66 % compared to 72–82 % for the rest of treatments, P < 0.05).
The aforementioned changes in soil properties caused by biochar, increase in soil field capacity and decrease soil compaction, must be less relevant under our experimental conditions. The soil used in the present study had a high percentage of sand (80 %, a higher proportion of macropores relative to fine-textured soils) and pots were irrigated daily, being soil moisture kept within the optimal range during the cropping experiment. However, the improvement of soil water-holding capacity by biochar addition could maintain a better moisture level between irrigation periods, being considered a key factor to obtain good grain yield in wheat (Gooding and Davies 1997). In addition, the effects of biochar addition on soil properties may have a significant effect under field conditions, where water availability and bulk density are important factors influencing plant growth, germination, and grain production.
The application of wheat straw biochar decreased total dry plant biomass, but in the case of olive tree pruning biochar it had no effect on total plant biomass (Table 1, Fig. 2). These changes were less noticeable than the increases resulting from the mineral fertilization. However, both biochars increased the grain percentage at the expenses of leaf, stem, and/or root reductions (Table 1). Both biochars did not have a statistical significant effect on the gas exchange variables (Table 1). In addition, wheat straw biochar increased the ear appearance and decreased the evapotranspiration; while olive tree pruning biochar decreased the chlorophyll content. Asai et al. (2009) related decreases in leaf SPAD values after biochar addition to a decrease in the soil N availability since this parameter is highly correlated to the nitrogen nutritional status of the leaf.
Biochar addition to soil generally increased wheat grain production in the absence of the mineral fertilization, ranging from −3 to 42 % compared to the control soil (Fig. 2). These increases were clearly lower than those caused by the use of mineral fertilization: 149 and 281 % compared to the control soil for the medium and complete mineral fertilization without biochar addition, respectively (Fig. 2). These results are in agreement with several authors (Chan et al. 2007; Van Zwieten et al. 2010), who found little responses of crop yield and nutrient status to the sole use of biochar, which are likely due to its nature: a carbon-rich but nutrient-poor material. Therefore, most of the studies have shown that the beneficial effects of the addition of biochar on crop production are most evident when biochar is combined with mineral fertilizers (Asai et al. 2009; Chan et al. 2007; Lehmann et al. 2003; Schulz and Glaser 2012; Van Zwieten et al. 2010). The detected increases in nutrient efficiency after biochar amending have been mainly related to a greater nutrient retention, minimizing nutrient losses; improvements in soil properties like increase in water-holding capacity, decrease in soil compaction, and liming effect leading to immobilization of contaminants or nutrient mobilizations; and enhancement in soil biological properties such as more favorable root environment, microbial activities favoring nutrient availability, etc. In the present study, a significant biochar×mineral fertilization interaction was observed for wheat grain production (Table 1). Thus, the highest wheat grain productions were obtained by combining both the highest biochar and mineral fertilization application rates (P < 0.001). These represented an increase of 407 and 328 % with respect to the control soil and of 33 and 22 % with respect to the control soil plus the highest mineral fertilization for the olive tree pruning biochar and the wheat straw biochar, respectively (Fig. 2). These results demonstrated that the biochar addition at the highest application rate increased grain yield with respect to the sole use of the mineral fertilization under the presence of an optimum mineral fertilization. This fact indicated that the effect of biochar depended on the soil fertility status.
Biochar affected plant nutrient uptake (Table 2) by increasing P and Mg (olive tree pruning biochar) or Zn and Cu (wheat straw biochar) and decreasing Cu (olive tree pruning biochar) or K, Ca, and Mg (wheat straw biochar). Both biochars decreased plant uptake of N, Na, Fe, and Mn. As for the aboveground plant nutrient concentration (data not shown), wheat straw biochar addition to soil increased P, K, Zn, and Cu.
Both biochars decreased N, Fe, and Mn aboveground plant concentrations. Aboveground Fe concentrations were within the sufficiency range in all the treatments (25–100 mg kg−1, Mengel and Kirkby 2001); while those of N were in the low range even in the mineral fertilizer treatments (1–2 %) according to Mengel and Kirkby (2001). The detected decreases in the aboveground N concentrations resulting from addition of biochar (0.3 % lower in the plants grown in biochar treatments) were consistent with the reduction in resin-extractable ammonium and nitrate (Table 2, Fig. 3). Indeed, the strong affinity of biochar for ammonium and nitrate has been previously reported (Atkinson et al. 2010; Lehmann et al. 2003; Mizuta et al. 2004; Taghizadeh-Toosi et al. 2012) and can avoid nutrient losses by leaching (Chan et al. 2007; Lehmann et al. 2003). In our experiment conditions, nutrient losses were absent since irrigation was controlled to avoid leaching, so this fact should not be relevant and biochar adsorption capacity might have limited nitrogen availability in the short term, especially after the mineral fertilizer addition. Asai et al. (2009) noted how biochar application may offset the effect of N fertilizer. Biochars characterized by a low nitrogen content, negligible content in inorganic forms and high C/N ratios can limit the availability of nitrogen for plants (Asai et al. 2009; Chan et al. 2007; Rajkovich et al. 2012). However, under field conditions, the fact that biochar addition can avoid nutrient losses by leaching may favor an increase in the availability of nutrients in soil in the long term.
Effects of biochar application rate and mineral fertilization level on resin-extractable soil nutrient concentration (mean value ± standard error, n = 5; where absent, bars fall within symbols). F0 without mineral fertilization, F40 mineral fertilization 40 %, and F100 mineral fertilization 100 % (complete). Biochar addition to soil had a significant effect on soil nutrient availability, decreasing resin-extractable nitrate and ammonium concentrations and increasing phosphate concentration. This reveals the great impact of biochar on the soil nutrient fertility status
It is remarkable that plants grown on the soils treated with high rates of biochar showed markedly lower aboveground Mn concentrations than those grown on the control soils; below 20 mg kg−1, limit value defined for Mengel and Kirkby (2001) as sufficient for wheat. This should be related to soil pH and the carbonate in the biochars since Mn deficiency is most likely to occur at high pH, typical of calcareous or over-limed soils. Even though N or Mn concentrations were low, plants did not exhibited visual signs of deficiency.
Both biochars seem to have a high nutrient retention capacity but the more intense decreases of aboveground Fe, Mn, Zn, and Cu concentrations for plants growing on soils amended with olive tree pruning biochar were probably due to the high soil pH increases and liming capacity induced by this type of biochar relative to wheat straw biochar. It has often been shown that the addition of biochar to soil increases the immobilization of nutrients and metal(loid)s through adsorption reactions (Beesley et al. 2011). However, due to the nature of the biochars used in this experiment, precipitation reactions may be relevant as an important process of metal(loid) immobilization in the presence of anions such as sulfate, carbonate, hydroxide, and phosphate when soil pH is high (Adriano 2001). Liming often increases the precipitation of metal(loid)s.
Interestingly, biochar addition had positive effects on plant P nutrition, being this result corroborated by the increase detected in the resin-extractable phosphate concentration after biochar amending. Biochar seems to represent a significant source of available P for crops (Asai et al. 2009; Lehmann et al. 2003). Atkinson et al. (2010) reviewed several mechanisms which can enhance availability and plant uptake of P after biochar addition to soil. It acts as source of soluble P salts and exchangeable P forms, avoids P precipitation by modifying soil pH (bonding or sorbing elements which precipitate P) or enhance microbial activity leading to changes in P availability.
4 Conclusions
Our results showed that the addition of biochar alone had a positive effect on some growth parameters but clearly lower when compared to the use of the mineral fertilization. In addition, there was a significant biochar×mineral fertilization interaction since the highest grain production was obtained when biochars were combined with the complete mineral fertilization, demonstrating the beneficial effect of biochar on wheat yield.
Biochar had much influence on soil properties, which can explain its effects on plant growth and grain production. Both biochars caused significant increases in soil pH, electrical conductivity, and resin-extractable phosphate. However, the application of biochar decreased resin-extractable ammonium and nitrate. The most relevant effect on plant nutrition was a decrease in N and especially in Mn plant uptake, which can limit crop performance in the long term. Therefore, the nutrient–biochar interactions should receive special attention.
In summary, biochar addition can enhance wheat yield with the environmental benefits of global warming mitigation, contributing to a more sustainable agriculture. The knowledge gathered from this type of studies is a key tool to implement pyrolysis as a management option for waste materials, guaranteeing both agricultural and environmental benefits.
References
Adriano D (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, Inoue Y, Shiraiwa T, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res 111:81–84. doi:10.1016/j.fcr.2008.10.008
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 33:1–18. doi:10.1007/s11104-010-0464-5
BAMP 2012. Bolsa andaluza de materias primas. http://www.bolsaandaluza.com/index/tda.php. Accessed 2 November 2012
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282. doi:10.1016/j.envpol.2011.07.023
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of green waste biochar as a soil amendment. Aust J Soil Res 45:629–634. doi:10.1071/SR07109
D’Angelo E, Crutchfield J, Vandiviere M (2001) Rapid, sensitive, microscale determination of phosphate in water and soil. J Environ Qual 30:2206–2209. doi:10.2134/jeq2001.2206
FAO 2012. Food and Agriculture Organisation. FAOSTAT database. http://faostat.fao.org/site/339/default.aspx. Accessed 2 November 2012
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agriculture use. Trans ASABE 51:2061–2069
Gooding MJ, Davies WP (1997) Wheat production and utilization: systems, quality and the environment. CAB International, Wallingford, UK
Hay RKM, Walker AJ (1989) Introduction to the physiology of crop yield. Longman Group UK, UK
Iliffe R (2009) Is the biochar produced by an anila stove likely to be a beneficial soil additive? MSc Diss. UK Biochar Research Centre Working Paper 4
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosys Environ 144:175–187. doi:10.1016/j.agee.2011.08.015
Lehmann J, da Silva JP, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357. doi:10.1007/s11104-007-9193-9
Mengel K, Kirkby EA (2001) Principles of plant nutrition. Kluwer, Dordrecht, The Netherlands
Mizuta K, Matsumoto T, Hatate Y, Nishihara K, Nakanishi T (2004) Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Biores Tech 95:255–257. doi:10.1016/j.biortech.2004.02.015
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeny RH (eds). Methods of soil analysis. Part 2. Chemical and microbiological properties. Agron. No. 9, 2nd ed. American Society of Agronomy, Madison, WI, pp 403–430
Pascual JA, García C, Hernandez T (1999) Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol Fertil Soils 30:1–6. doi:10.1007/s003740050579
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils 48:271–284. doi:10.1007/s00374-011-0624-7
Schulz H, Glaser B (2012) Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J Plant Nutr Soil Sci 175:410–422. doi:10.1002/jpln.201100143
Sims GK, Ellsworth TR, Mulvaney RL (1995) Microscale determination of inorganic nitrogen in water and soil extracts. Comm Soil Sci Plant Anal 26:303–316. doi:10.1080/00103629509369298
Sohi SP, Krull E, López-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82. doi:10.1016/S0065-2113(10)05002-9
Stavi I, Lal R (2013) Agroforestry and biochar to offset climate change: a review. Agron Sustain Dev 33:81–96. doi:10.1007/s13593-012-0081-1
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69. doi:10.1007/s11104-011-0870-3
TMECC (2002) Test methods for the examination of composting and compost. U.S. Composting Council, Bethesda, MD
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. doi:10.1007/s11104-009-0050-x
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:689–696
Wintermans JFGM, de Mots A (1965) Spectrophotometric determination of chlorophyll a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453
Zucconi F, Pera A, Forte M, de Bertoldi M (1981) Evaluating toxicity of immature compost. Biocycle 22:54–57
Acknowledgments
This research was funded by the Spanish “Ministerio de Ciencia e Innovación” (Programa Nacional de Cooperación Público-Privada, Subprograma INNPACTO) and FEDER Funds “Fondo Europeo de Desarrollo Regional, una manera de hacer Europa” in the framework of the project “Proyecto Biocar: Estudio del Biocarbón como Sumidero de Carbono” (IPT-440000-2010-8). The authors would like to thank M. Olmo, C. Allely, M.A. Rey, M. Castro, J.C. Cañasveras, and A.R. Sánchez-Rodríguez for their help in conducting the experiment. We also thank L. Gómez and A. Serrano from Zero Emissions Technologies S.A. for their contribution to the project development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Alburquerque, J.A., Salazar, P., Barrón, V. et al. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 33, 475–484 (2013). https://doi.org/10.1007/s13593-012-0128-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13593-012-0128-3