Abstract
Hygienic behavior in honey bees, Apis mellifera, has been studied for over 80 years with the aim of understanding mechanisms of pathogen and parasite resistance and colony health. This review emphasizes the underlying behavioral mechanisms of hygienic behavior in honey bees and when known, in other social insects. We explore the relationship between honey bee hygienic behavior toward diseased brood and Varroa-parasitized brood (Varroa-sensitive hygiene, VSH); the timing of hygienic removal of diseased, Varroa-infested, and virus-infected brood relative to risk of transmission that can affect colony fitness; and the methods, utility, and odorants associated with different assays used to select colonies for resistance to diseases and Varroa. We also provide avenues for future research that would benefit honey bee health and survivorship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hygienic behavior is an important form of social immunity (Cremer et al. 2007) for a number of social insect species. The term hygienic behavior was coined by Rothenbuhler (1964) to describe the process of detection and elimination of diseased brood by adult honey bees (Apis mellifera). The term “Varroa-sensitive hygiene” (VSH) was coined more recently (Harris 2007) to describe the detection and removal of brood infested with the parasitic mite Varroa destructor by honey bees (Harbo and Harris 2005). The behavioral sequence of uncapping and removing the brood, as first described (Rothenbuhler 1964), is the same whether the brood is diseased, mite-infested, or dead, but this motor pattern may be triggered by the detection of different odorants associated with the health status of the brood. In honey bee colonies, elimination of brood consists of adult bees removing and/or cannibalizing the abnormal brood from individual cells, either intact or in pieces, and discarding remains outside the hive; in Reticulitermes termites, it consists of cannibalization (Davis et al. 2018) and in Lasius ants of destructive disinfection by dismembering the infected pupa and then disinfecting with venom (Pull et al. 2018). Hygienic behavior helps maintain the health of densely populated insect societies by limiting horizontal transmission of pathogens and population growth of parasites. Workers that destructively eliminate already infected or infested individuals protect the colony, or superorganism, in a similar way to immune cells that protect an organism from pathogen spread throughout the body (Cremer and Sixt 2009).
In recent years, research on hygienic behavior in honey bees has increased with the aim of understanding and restoring colony health. The early research on this behavior was in relation to honey bee resistance to American foulbrood (caused by Paenibacillus larvae) and to chalkbrood (caused by Ascosphaera apis) diseases (Spivak and Gilliam 1998a, b). Focus shifted to the relationship between hygienic behavior and resistance to the parasitic mite, Varroa in the 1990s (Leclercq et al. 2018a; Mondet et al. 2020). This review emphasizes the underlying behavioral mechanisms of hygienic behavior in honey bees and when known, in other social insects. The goals of this review are to (1) explore the relationship between honey bee hygienic behavior toward diseased brood and Varroa-parasitized brood and (2) provide avenues for future research that would benefit honey bee health and survivorship.
2 Timing of hygienic removal of diseased brood
In the 1930s, the most serious disease of honey bees was American foulbrood. Beekeepers and researchers (Park 1937) noted that some colonies did not succumb to this disease and they considered these colonies to be resistant. They observed that “the bees sometimes remove and dispose of larvae very soon after they die, thus eliminating the evidence.” Following these observations, it was determined that first instar larvae derived either from resistant or from susceptible colonies were equally susceptible to American foulbrood, but larvae inoculated more than 2 days and 5 h after hatching from the egg did not become infected (Woodrow 1942). It was later observed that the adult bees from resistant colonies removed the majority of the diseased brood from the cells whereas bees from susceptible colonies did not, and concluded that colony resistance depended on behavioral removal of diseased brood by adult bees, rather than physiological resistance of the brood (Woodrow and Holst 1942). These findings were later confirmed by Rothenbuler and others (Spivak and Gilliam 1998a).
The experiments by Woodrow and Holst also revealed that the timing of adult bees’ removal of the infected brood was key to understanding the apparent resistance (Woodrow and Holst 1942). After suspending known quantities of P. larvae spores in the food surrounding individual first instar larvae, they noted that the resistant colonies started eliminating the infected larvae on the sixth day after inoculation (the day the cell containing a 5th instar is capped with wax) and had removed all of infected brood by day 11. They collected intact brood that was removed from the hive by the bees and found the brood had only the non-infectious rod form of P. larvae, indicating the bees were removing the brood from the nest while the non-infectious rods were multiplying within them. In contrast, bees in a susceptible colony did not begin removing infected brood until day 9 after inoculation, and not all of the diseased brood was removed from the cells; some was uncapped but later recapped with wax. The bacterium reached the highly infectious spore stage in the remaining brood of the susceptible colonies, and bees from susceptible colonies were sometimes removing the brood while bacteria were infectious, potentially spreading the disease. Woodrow and Holst concluded that “…resistance to American foulbrood in the honey bee colony consists in its ability to detect and remove diseased brood before the causative organism… reaches the infectious spore stage in the diseased larvae.” Observations of hygienic activity against brood infected with Mellisococcus plutonius prompted J. I. Hambleton to report that “American foulbrood resistant strains are highly susceptible to European foulbrood” (Root 1966). The apparent susceptibility may have been because the bees were actively handling younger honey bee larvae which have infectious M. plutonius but non-infectious P. larvae; this possibility requires further study.
The timely detection and removal of brood was demonstrated after bees were challenged with a different pathogen, the chalkbrood fungus (Invernizzi et al. 2011). The most hygienic colonies, those that uncapped pin-killed brood (see section on “Assays” below), also tended to uncap cells and cannibalize the chalkbrood-infected brood before the brood was consumed by fungal mycelia and became infectious “mummies.” Colonies with numerous intact chalkbrood mummies on the bottom board of the colony indicated that bees were not hygienic because the infected brood was removed after it reached the spore stage, increasing the risk of horizontal transmission.
The timely elimination of infected brood is important for other social insects, such as colonies of the invasive garden ant, Lasius neglectus (Tragust et al. 2013; Pull et al. 2018), and the subterranean termite, Reticulitermes flavipes (Davis et al. 2018). These social insect colonies nest in the soil where they may become exposed to the soil-borne fungal entomopathogens such as Metarhizium. Adult ants in the genus Lasius groom infectious conidiospores of Metarhizium from the brood into infrabuccal pouches and disinfect the fungal pellets in their pouches with their antimicrobial venom (Tragust et al. 2013). If the fungus is undetected on the cuticle of some ants and germinates into the pupal body, upon detection of the infected pupa, the adult ants unpack it from its cocoon, dismember it, and disinfect the pupal remains with venom (Pull et al. 2018). The detection and destructive disinfection of the infected pupa occurs when the pathogen is in the non-infectious incubation period, similar to how honey bees detect and remove infected, but not infectious pupae from the nest. The destructive disinfection prevented the pathogen from completing its life cycle, thus preventing intra-colony disease transmission (Pull et al. 2018). In R. flavipes termite colonies, Metarhizium conidiospores are groomed from infected individuals, but once the fungus enters the body, termites cannibalize the infected nestmate (Davis et al. 2018). It was not determined if cannibalism occurred during the non-infectious incubation period; however, the switch from sanitary prevention (allo-grooming) to elimination (cannibalism) was clear, suggesting that termites also are able to detect the stage of infection (Davis et al. 2018).
In sum, the timing of detection and elimination of the diseased brood by adult social insects seems to be a critical component in preventing pathogen transmission within these social insect colonies, and thus in colony-level resistance. It would be to the pathogen’s advantage for individuals within the colony to handle diseased brood when infectious because it would increase the risk of pathogen transmission, whereas it would be to the colony’s advantage if individuals eliminate the brood before it is infectious because it would limit pathogen spread. Whether the timing of the elimination of brood when mite-infested is similarly important is discussed below.
3 Assays for honey bee hygienic behavior
Bioassays for hygienic behavior were recently reviewed in depth (Leclercq et al. 2018a) and thus, only some points are highlighted here. The best way to determine if a colony of honey bees (or other social insects) can detect and remove diseased brood is to challenge individual bees or larvae, or an entire colony, with a known dose of a pathogen and observe the response of adult nestmates to infected individuals. Due to the risks involved in challenging honey bee colonies with potentially lethal and highly infectious pathogens such as P. larvae, researchers began exploring assays that would not involve inoculating larvae with a pathogen. As a proxy for diseased brood, cyanide-killed brood was presented in colonies to facilitate experiments using lines of bees already selected for resistance and susceptibility to American foulbrood (Jones and Rothenbuhler 1964). Later, researchers began screening unselected colonies for hygienic behavior using freeze-killed brood (Spivak and Gilliam 1998b), or pin-killed brood (Newton and Ostasiewski 1986).
How quickly a colony could detect and remove the experimentally killed brood did not always correspond with the colony’s ability to remove diseased brood (Gilliam et al. 1983). Thus, after screening colonies using a freeze-killed (or pin-killed) brood assay, it is important to subsequently challenge colonies with a pathogen to determine if they are behaviorally resistant (Spivak and Reuter 2001a). As a recent example, an imperfect correspondence was found between the removal of freeze-killed brood and physiological resistance to chalkbrood in Australian honey bee colonies (Gerdts et al. 2018). Of 649 colonies tested for hygienic behavior using the freeze-killed brood assay, 16% were considered highly hygienic (removed 95% of the freeze-killed brood within 24 h), suggesting they should not have signs of disease within the colony, but in fact, 23% of these highly hygienic colonies presented signs of chalkbrood disease. These results provide an example of how the freeze-killed brood assay does not fully predict behavioral resistance in the test population.
Of note is that colonies that remove less than 95% of the freeze-killed brood within 24 or 48 h tend to remove little, if any, pathogen infected brood after challenge; they tend not to be resistant to American foulbrood or chalkbrood (M Spivak, unpublished data). This observation begs the question of why highly hygienic colonies are rare in nature and whether there are associated fitness costs with the trait (Spivak and Gilliam 1993; Mondragon et al. 2005; Bigio et al. 2014; Leclercq et al. 2017). We speculate that resistance does not depend solely on hygienic behavior but likely involves a combination of other physiological factors in honey bees, including the immune response (Evans and Spivak 2010), transgenerational immune priming (Hernandez Lopez et al. 2014), microbiome community (Raymann and Moran 2018), antimicrobial activity of larval food (Rose and Briggs 1969; Borba and Spivak 2017), presence of propolis in the nest (Borba et al. 2015), and other factors yet to be discovered.
In sum, and as pointed out previously (Leclercq et al. 2018b), assays for hygienic behavior, like the freeze-killed or pin-killed brood assays, are not necessarily useful predictors of pathogen resistance in a colony or population of colonies. They are useful to screen colonies for the ability of the adult bees to quickly remove dead brood (e.g., > 95% removal within 24 h for the freeze-killed brood test) and these colonies can be subsequently challenged to quantify pathogen resistance. In other words, the assays are used to narrow down the number of colonies to be challenged, to increase the chances of finding resistant colonies.
4 Hygienic behavior in relation to Varroa
Although some ant and termite colonies have brood parasites (Korb and Fuchs 2006; Lachaud et al. 2016), studies of their hygienic response are limited; e.g., the ant Ecatomma tuberculatum detects and removes parasitic wasps (Perez-Lachaud et al. 2015) and other nest intruders (Perez-Lachaud et al. 2019). Thus, this section will concentrate on honey bees’ response to Varroa destructor. When V. destructor spread through A. mellifera colonies in Europe and North America, researchers looked to this mite’s original host species, A. cerana, to determine how it survived without succumbing to the parasite. A number of potential resistance mechanisms were described (Boecking and Spivak 1999), hygienic behavior being one of them (Peng et al. 1987a; Peng et al. 1987b; Rath 1999). In Apis cerana, Varroa reproduces only on seasonally produced drone brood and does not reproduce on worker brood. If the mite infests worker brood (or are experimentally introduced onto worker pupae), the pupa dies, due to a toxic salivary gland secretion injected by mite (Zhang and Han 2018) and the bees hygienically remove the dead brood from the nest (Page et al. 2016). The signal or cue from the dying pupa was termed “altruistic suicide” and the removal “social apoptosis”; the combination was hypothesized to increase inclusive fitness benefits to the colony (Page et al. 2016). In A. mellifera, Varroa reproduces successfully on both drone and worker brood, and worker pupae do not die if infested with the mites, although they could if also infected with high enough virus levels (Martin 2001; de Miranda and Genersch 2010; Dainat et al. 2012).
After Varroa spread through Europe, A. m. carnica colonies in Germany were tested for their ability to detect and remove Varroa-infested brood (Boecking and Drescher 1992). The removal of infested brood would be a form of mite resistance because it would increase mite mortality or disrupt mite reproductive success (Leclercq et al. 2018a). In the Boecking and Drescher study (Boecking and Drescher 1992), the colonies were not previously selected for hygienic behavior or mite resistance. After experimentally introducing mites into recently capped brood cells, 29% of the infested brood were removed after 10 days when one mite per cell was introduced and 55% were removed when two mites per cell were introduced, indicating that in fact, some A. mellifera colonies could detect and remove some mite-infested pupae, even though they were naïve hosts to this parasite.
Later, it was explored whether colonies selected for hygienic behavior based on the freeze-killed brood assay would be able to detect and remove pupae infested with Varroa (Spivak 1996). Two lines of bees were challenged with Varroa, one bred over several generations for rapid hygienic behavior (colonies that removed > 95% of the freeze-killed brood within 48 h, the Minnesota Hygienic bees) and one bred for slow hygienic behavior (colonies that removed 20–30% of freeze-killed brood within 48 h). Thus, the test population was more bimodal than continuous in its hygienic response. In 3 of 4 years, the rapid or highly hygienic colonies removed over 60% of the experimentally mite-infested brood by the 10th day after one mite per cell was introduced into recently sealed brood. The slow or non-hygienic colonies removed 10–20% of the infested brood in the same years (Figure 1).
The mean (± s.e.) percentage removal of mite-infested pupae by hygienic and non-hygienic colonies 10 days after introduction of one mite per cell through cell bases (Spivak and Gilliam 1998b). In 1994, 1996, and 1997, the hygienic colonies (n = 4, 10, and 6 respectively) removed significantly more pupae infested with one mite per cell than did the non-hygienic colonies (n = 3, 6, and 6) (P < 0.01; split-plot two-way ANOVA for each year). There was also a significant difference between the removal of infested pupae and controls (P < 0.05) in those years. Tests in 1995 (n = 7 hyg, 4 non-hyg) revealed a significant difference only when two mites per cell were introduced (data not shown; treatment effect: P < 0.01).
A significant negative relationship between the results of the freeze-killed brood assay and mite population growth over one season was found in the UK (Toufailia et al. 2014). The statistical significance was driven by eight of the 42 colonies that removed > 95% of the freeze-killed brood within 48 h and were thus highly hygienic, again confirming that screening for these highly hygienic colonies based on the freeze-killed brood assay will help locate colonies with a relatively higher potential of removing mite-infested brood. Colonies that removed less than 95% of the freeze-killed brood showed no significant relationship between hygienic behavior and mite growth (Toufailia et al. 2014), which was also observed in Mexico (Mondragon et al. 2005).
A large population derived from diverse sources of colonies in western Canada was selected over three generations for hygienic behavior using either the freeze-killed brood test or peptide biomarkers from bees’ antennae, with the goal of testing the utility of marker-assisted selection for hygienic behavior (Guarna et al. 2017). Eleven of the 13 protein markers were linked to hygienic behavior (including two linked to VSH, see section below), and two were linked to grooming behavior. This remarkable study showed two things: that protein biomarkers can be used successfully in breeding bees (and possibly other livestock) and that compared to unselected stocks, colonies selected using either the freeze-killed brood assay or peptide biomarkers had increased hygienic behavior, showed no loss of honey production, and had increased survival when challenged with either P. larvae or Varroa.
Researchers in Germany have used the pin-killed brood assay in breeding programs to successfully reduce mite loads. Other researchers reported no correlation between the removal of freeze-killed or pin-killed brood and the mite infestation of colonies, reviewed in Locke (2016). However, the latter studies used these assays to try to determine the mechanism of resistance of a population, not to screen and narrow down the number of colonies for subsequent challenge to quantify potential resistance, or to use in breeding programs.
In sum, the freeze-killed and pin-killed brood assays for hygienic behavior are useful screening tools to find colonies that may remove diseased and mite-infested brood upon subsequent challenge. For Varroa in particular, selecting bees based on these assays will yield colonies with lower mite loads relative to unselected colonies (Spivak and Reuter 1998; Spivak and Reuter 2001b; Büchler et al. 2010; Guarna et al. 2016; Guarna et al. 2017) but to date, selection using these assays has not resulted in populations resistant to mites; that is, populations that do not require treatment to survive. Thus, these field assays should not be used as sole tests or indicators of Varroa resistance, as other traits contribute to various degrees to this resistance, reviewed in Mondet et al. (2020).
5 Varroa -sensitive hygiene
Varroa-sensitive hygiene is a specialized term for the hygienic trait in which honey bees detect and remove brood specifically infested with Varroa. VSH activity is largely the same as that of the hygienic trait; the bees perform the hygienic behavioral sequence of uncapping and removing brood, but the removal in this case is triggered by the detection of mite-infested brood, rather than diseased or dead brood. Note that the term VSH also is often used for lines of bees bred for enhanced expression of the trait. Bees that express high levels of VSH show clear resistance to Varroa in that they do not require treatments to survive mite infestations, as has been demonstrated by USDA researchers in Baton Rouge, LA, USA. Of note, a critical experiment has not been conducted which could clarify the relationship between colonies selected for VSH and those selected for hygienic behavior based on the freeze-killed or pin-killed brood assay. It would be informative to challenge colonies that express VSH with P. larvae or A. apis pathogen to determine if bees that express VSH only respond to mite-infested brood, or if they also detect and remove diseased brood and thus, are hygienic in general.
This history of bees with VSH-based mite resistance, and how it has been selected over the years is somewhat convoluted. Harbo and Hoopinger began by searching for colonies that displayed resistance to Varroa with no a priori assumptions about which traits would be involved (Harbo and Hoopingarner 1997). They inoculated 43 colonies with known quantities of Varroa at the beginning of the season and quantified mite loads after ~ 10 weeks. They found three colonies with fewer mites at the end of the test than were originally inoculated. After running a number of tests to determine the mechanism for active resistance against the mites, they concluded that the factor that best explained the apparent resistance was the low reproductive success of the mites on worker brood. They selectively bred a line from several of the highest-performing colonies and gave it the name suppression of mite reproduction or SMR. The mechanism for how bees or brood from the SMR colonies could reduce mite reproductive success was unknown. The mites entered worker brood cells to feed and reproduce; however, the authors reported that the mites died in the cell without reproducing, produced no progeny, produced males only, or produced progeny too late to mature (Harbo and Harris 1999).
SMR colonies removed > 95% of the freeze-killed brood within 48 h, which indicated that the bees were expressing a high level of hygienic behavior (Ibrahim and Spivak 2006). These results were surprising because the SMR line was selectively bred for reduced mite reproduction, not for hygienic behavior (Harbo and Harris 1999). It was hypothesized that the SMR bees could be detecting and removing pupae on which the mites were reproducing, leaving pupae with mites that did not reproduce successfully. This hypothesis was tested in two ways. In one test, recently capped brood combs with known percentages of mite infestation were introduced into colonies with and without the SMR trait (Harbo and Harris 2005). After 8 days, the SMR colonies had significantly lower mite infestation (2%) compared to the controls (9%). Of the mites that remained, the SMR colonies had a lower proportion of reproductive mites, 20% vs. 71%, suggesting the SMR bees were targeting pupae with reproductive mites. In a second test, mites were experimentally introduced onto individual pupae of two types of colonies: SMR bees and Minnesota Hygienic bees that had been selected using the freeze-killed brood assay (Ibrahim and Spivak 2006). The SMR colonies removed significantly more mite-infested pupae than colonies from the hygienic line. Together, these findings indicated that bees bred for SMR express hygienic behavior and that adult bees may selectively remove pupae infested with reproductive mites. In addition, hygienic activity may disrupt the reproduction of mites on targeted pupae (Kirrane et al. 2011), and some of these mites may re-invade other open brood cells and later be counted as non-reproductive. In 2007, Harris renamed the line from SMR to VSH to reflect that the main mechanism that leads to non-reproductive mites (and thus mite resistance) apparently is hygienic behavior rather than the ability of the brood to somehow reduce mite reproductive success.
A further finding was that the reproductive success (fertility and number of viable female offspring) of Varroa on pupae not hygienically removed by bees was significantly lower in VSH colonies than in Minnesota Hygienic colonies (Ibrahim and Spivak 2006). This suggests an additional effect of VSH pupae that reduced mite reproductive success, indicating that hygienic behavior alone was not completely responsible for the mite resistance in this line. Recent studies also suggest a brood effect that suppresses mite reproduction (Wagoner et al. 2018; Wagoner et al. 2019). Such an effect originating from brood could be a valuable trait to support mite resistance. However, a brood-based effect was not increased reliably in an attempt to select and breed for it (Villa et al. 2016).
The methods used for selecting Varroa-resistant bees by the USDA researchers in Baton Rouge has varied through time. Progress originally came by quantifying the relative population growth of the mites over a short period, typically ~ 10 weeks (Harbo and Hoopingarner 1997). Colonies later were selected based on the frequency of non-reproductive mites in them, after this factor was determined to be the principal determinant of resistance (Harris and Harbo 2000). The frequency of non-reproductive mites has been the most extensively used criterion for selection and continues to be used today. After the role of hygiene was discovered, some selection involved introducing combs containing known percentages of mite-infested brood and quantifying the decrease in infestation after 1 week (Villa et al. 2009). This method requires more replication to be accurate when colonies being tested have low mite resistance (Villa et al. 2017). Experience with these three methods suggests that highly mite-resistant colonies (i.e., those that require no treatment against Varroa) generally have mite population growth of ≤ 1.0 per reproductive cycle (Harbo and Harris 2009) and ≥ 60% of mites that are non-reproductive, and remove ≥ 80% of mite-infested brood after 1 week (Danka et al. 2016).
Measuring mite population growth, the frequency of non-reproductive mites or the removal of mite-infested brood is technically difficult and tedious, and these issues have limited bee breeders’ selection for the VSH trait. To date, there is no simple field assay that will yield the high Varroa resistance of the bees selected with these technical methods. Selection based on the freeze-killed brood assay will not be sufficient (Danka et al. 2013) (and discussed earlier). Some resistant populations, particularly the “survivor” stocks that thrive without treatment indicate that hygienic behavior, however assayed, may not be the main mechanism for all populations, e.g., African populations in Africa and the neotropics, plus populations in Sweden, France, and the Arnot Forest in New York (Locke 2016; Mondet et al. 2020). Populations of highly resistant bees, including survivor populations (Locke 2016) and Russian bees (Rinderer et al. 2001), display non-reproduction of mites or low mite population growth, but the lack of, or slow, mite increase may be due to a combination of inter-related factors that range from life-history traits (e.g., high swarming frequency) to distinct behavioral traits (VSH or grooming).
6 Timing of removal of Varroa-infested and virus-infected brood
It is not known if the timing of detection and removal of Varroa-infested brood is as critical of a component in preventing parasite transmission as it is for pathogen transmission during removal of diseased brood. This issue has not been studied. The timing of hygiene may not depend on the presence of the mite per se but on the virus levels in the pupae, such as deformed wing virus (DWV), which are induced to replicate and vectored by the mites as the mite feeds. The bees’ removal of mite-infested brood tends to increase 72 h after the larvae is capped with wax (Spivak 1996; Harris 2007), which is when the larva initiates metamorphosis into a pupa and when the mite feeds and begins reproducing in the cell (Donzé and Guerin 1994; Martin 1995; Donzé and Guerin 1997). The removal process can continue for the duration of pupal development (Vandame et al. 2002). Hygienic handling of the virus-infested brood could either increase or decrease transmission of the pathogen. The risk of increasing transmission would depend on the type and level of the virus infection, which could depend on the stage of bee pupal development, and the relative infectivity and virulence of the virus to the bees. This area requires testing because these factors are only beginning to be understood in honey bees (Brutscher et al. 2016; Grozinger and Flenniken 2019).
A few studies have shown a link between hygienic behavior and reduction in virus-infested brood. Hygienic colonies, determined based on the pin-killed brood assay, tended to remove worker pupae infected with DWV (Schöning et al. 2012). Highly hygienic colonies, determined based on the freeze-killed brood assay, also had significantly lower levels of DWV in addition to lower mite population growth over the season (Toufailia et al. 2014). Brood infected with DWV produced chemical compounds that when experimentally applied to brood elicited hygienic behavior (Wagoner et al. 2019). The correspondence between mite infestation, virus load, and stimulus intensity has not been explored relative to the timing of hygienic detection and removal by honey bees. Understanding the relationship among these factors will not be easy, nor necessarily robust from one population of bees to the next, but is worthy of study.
7 Mechanisms of detection of diseased and Varroa-infested brood by adult bees
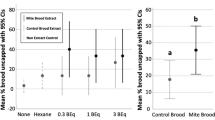
To study the mechanisms underlying how adult honey bees detect diseased brood before the pathogen reaches the infectious spore stage, it was hypothesized that hygiene was mediated by olfactory stimuli emitted from diseased brood (Spivak et al. 2003). It was not known if the odorant was passively or actively emitted, i.e., whether it was a cue or signal (Maynard Smith and Harper 2003; Leonhardt et al. 2016). A number of neuroethological methods were employed to test the olfactory hypothesis, using chalkbrood as the test pathogen, and the line of honey bees selectively bred for hygienic behavior based on the colony response to a freeze-killed brood assay (Arathi et al. 2000; Masterman et al. 2000; Masterman et al. 2001; Gramacho and Spivak 2003; Spivak et al. 2003; Arathi et al. 2006; Swanson et al. 2009). Based on the results of these experiments, it was concluded that bees from hygienic colonies were able to detect and discriminate between odors of diseased and healthy brood at a lower stimulus level compared to bees from non-hygienic colonies. Non-hygienic bees would, and do, detect and remove diseased brood, but only when the pathogen is infectious and the stimulus level is very high (Figure 2), increasing the risk of pathogen transmission.
Similarly, observations by USDA scientists revealed different reactions to Varroa infestation from bees with VSH-based hygiene versus from bees that were not selected for hygiene. Unselected bees tended to detect and remove dead or highly diseased brood but not live, mite-infested brood on the same comb. Exposing the same brood comb to VSH bees, however, resulted in hygiene against mite-infested brood that the unselected bees ignored (Figure 3). This example illustrates how hygienic responses to mite-infested brood and disease-infected brood both vary depending on the olfactory sensitivity of the adult bees.
Different hygienic responses to unhealthy brood by unselected colonies and mite-resistant colonies. a Dead, Varroa-infested and diseased prepupae that were hygienically uncapped in a colony of unselected bees; b the same comb after all unsealed brood had been manually removed, and the comb then had been exposed for 2 h in a colony with high expression of VSH; c live, Varroa-infested pupae being uncapped and removed in the mite-resistant colony.
A complementary approach to quantifying olfactory sensitivity of adults is to identify odorants emitted by the brood when dead, diseased, or parasitized. There have been several studies that attempted to identify the compounds emitted by mite-parasitized brood that elicit hygienic removal of the brood (Nazzi et al. 2004; Schöning et al. 2012; Mondet et al. 2016; Wagoner et al. 2019). To date, results indicate there may be multiple compounds associated with Varroa-infested or disease-infected brood, which may vary as a function of the genetic origin of the bees (Wagoner et al. 2018) or of the experimental methods used by different researchers (reviewed in Mondet et al. (2020)).
The capability of A. mellifera to respond to live, mildly injured brood is seen when pupae are uncapped because of infestation by wax moth (Galleria or Achroia spp.) larvae. This activity suggests that a behavioral defense which predated Varroa is now being used as a primary tool of social immunity against the mite (Villegas and Villa 2006; Martin et al. 2019). The activity also is notable because it apparently represents a response only to stimuli produced by the pupa. Such stimuli (rather than stimuli from the wax moth larva) are suggested because the uncapping occurs for pupae within a narrow age range (typically those with white, pink, or purple eyes). These pupae are the same age as those targeted by hygiene against Varroa, suggesting that hygiene is closely related to pupal development. Stimuli initiated by a response of mildly injured or “disturbed” brood may be quantitatively and qualitatively different from stimuli produced from dead or severely injured or diseased brood. These latter stimuli may be associated with broader hygienic responses rather than more narrowly targeted hygiene such as VSH.
It is known that social insects can detect sick brood while not infectious, but it is not known if the intensity of the stimulus (cue or signal) is low when detected and eliminated, and then increases in intensity as it reaches the infectious stage. The correspondence between disease load and stimulus intensity has been studied in ants. In Lasius, changes were found in the cuticular hydrocarbon profiles of ants infected with the fungal pathogen Metarhizium (Pull et al. 2018). Infected ant pupae had higher relative abundances of four cuticular hydrocarbons compared to uninfected control pupae, and infected pupae that were unpacked by adults had higher relative abundances of those four plus an additional four cuticular hydrocarbons compared to control pupae, suggesting that these compounds may increase in abundance over the course of infection. The compounds on the surface of the ants were long-chained cuticular hydrocarbons (carbon chain length C33–35) with low volatility and were distinct chemically from the compounds that induce undertaking, or the removal of corpses in ants (Wilson et al. 1958). Two of the four cuticular hydrocarbons that were increased on infected pupae had higher abundances on virus-infected honeybees (Baracchi et al. 2012) and were similar to the compounds found by Wagoner et al. (2018, 2019) on infected honey bee pupae that were detected and removed by hygienic bees. It was speculated that these compounds may be “conserved sickness cues” in social insects, selected over evolutionary time to enhance inclusive fitness of the diseased individual and to enhance direct fitness of the colony (Pull et al. 2018).
An untested hypothesis about the role of brood odorants that we propose is that adult honey bees may have a chemical recognition template of “healthy brood” and thus are able detect numerous compounds coming from any “abnormal” brood, whether dead, diseased, or parasitized. Specific odorants, however, may initiate responses that can be differentiated into hygienic response to pathogens or mites. This “healthy brood” template would be analogous to how the immune system detects self vs foreign using pattern recognition receptors (Medzhitov and Janeway 2002; Kawasaki and Kawai 2019) or how social insects recognize nestmates from non-nestmates using cuticular hydrocarbons (Perez-Lachaud et al. 2015; Leonhardt et al. 2016). The stimulus from healthy brood would likely be a blend of chemical compounds that vary with the age of the brood (Le Conte et al. 1990; Le Conte and Hefetz 2008; Mondet et al. 2020). Adult bees from hygienic colonies, while inspecting larvae and wax-capped brood with their antennae, would be able to detect and discriminate healthy from any abnormal brood because of different brood odorants. The specific chemical nature of the odorants of the abnormal brood may be less important to the bees compared to its relative irregularity from the healthy brood template.
8 Recapping
Colonies that are apparently resistant to Varroa display high frequencies of cell recapping; that is, the wax capping over a pupa is opened, then recapped with wax (Boecking and Spivak 1999; Aumeier et al. 2000; Harris et al. 2012; Oddie et al. 2018; Martin et al. 2019). Recapping by the bees is evident when the cell capping is experimentally removed and inspected from underneath; if it has been recapped, there is a circular area with no silk lining. It was postulated that uncapping and recapping of brood cells would disrupt mite reproduction in various ways (Leclercq et al. 2018a). Uncapping and recapping has been noted since early studies on resistance to American foulbrood (Woodrow and Holst 1942) and thus, this behavior is not specific to mite-infested brood. Recapping has been observed in relation to the removal of freeze-killed brood (Spivak and Gilliam 1993) and the process of uncapping and recapping was interpreted as inefficient task partitioning among bees of different hygienic tendencies (Arathi et al. 2006). Bees that uncapped the brood were not the same as those that removed it: the uncappers had higher olfactory sensitivity compared to the removers (Gramacho and Spivak, 2003). These data suggest that some bees may be detecting the stimuli from the dead (diseased or mite-infested) brood and chew a hole in the cell capping. Other bees with lower olfactory sensitivity may recap the cell, not detecting the problem within. At the other extreme, excessive uncapping is seen in some colonies where the brood appears to be healthy; this situation may indicate dysfunction between the components of hygiene (Figure 4).
The uncapping-recapping behavioral sequence requires further study, particularly in relation to mite infestation, where its function and utility as an indicator of hygienic behavior or mite resistance is still unclear (van Alphen and Fernhout 2019; Oddie et al. 2019). It is likely that the amount of recapping reflects the interplay between brood stimulus intensity and adult bee olfactory sensitivity of the test colonies, and may involve differences of olfactory sensitivities among patrilines within colonies.
9 Conclusions
-
1
The freeze-killed and pin-killed brood assays for hygienic behavior are useful screening tools to find colonies that may remove diseased and mite-infested brood upon subsequent challenge with a specific pathogen or Varroa; however, these field assays should not be used as sole tests or indicators of pathogen or Varroa resistance. Selection using these assays for hygienic behavior has not resulted in populations resistant to mites; that is, populations that do not require treatment to survive.
-
2
It would be helpful to clarify terms, for example, when Varroa-sensitive hygiene (VSH) is being referred to as a trait vs a bred line of bees. Referring to VSH as distinct trait, different from hygienic behavior (sometimes abbreviated HYG) is more confusing than helpful as they involve the same behavioral sequence of detecting, uncapping, and removing. The difference is in specificity: VSH refers to hygienic behavior directed to mite-infested brood. Hygienic behavior is a more general term for removal of dead, diseased (including virus-infected), and mite-infested brood. However, the critical test of challenging colonies bred for VSH with a pathogen to determine the specificity of the response has not been conducted.
-
3
Research is required on the timing of hygienic removal of Varroa-infested and virus-infected brood relative to risk of further virus transmission. More work is needed on the potential correspondence between brood stimulus intensity and level of pathogen infectivity in relation to hygienic detection and removal of the infected brood. It increases colony fitness when individuals eliminate the brood before it is infectious by limiting pathogen transmission; it may decrease colony fitness to handle diseased brood when infectious, depending on the infectivity of the pathogen and the health status of the colony.
References
Arathi HS, Burns I, Spivak M (2000) Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): Behavioural repertoire of hygienic bees. Ethology 106:365-379. https://doi.org/10.1046/j.1439-0310.2000.00556.x
Arathi HS, Ho G, Spivak M (2006) Inefficient task partitioning among nonhygienic honeybees, Apis mellifera L., and implications for disease transmission. Anim Behav 72:431-438. https://doi.org/10.1016/j.anbehav.2006.01.018
Aumeier P, Rosenkranz P, Goncalves L (2000) A comparison of the hygienic response of Africanizied and European (Apis mellifera carnica) honey bees to Varroa-infested brood in tropical Brazil. Genet Molec Biol 23:787-791
Baracchi D, Mazza G, Turillazzi S (2012) From individual to collective immunity: the role of the venom as antimicrobial agent in the Stenogastrinae wasp societies. J Insect Physiol 58:188-193. https://doi.org/10.1016/j.jinsphys.2011.11.007
Bigio G, Al Toufailia H, Ratnieks FL (2014) Honey bee hygienic behaviour does not incur a cost via removal of healthy brood. J Evol Biol 27:226-230. https://doi.org/10.1111/jeb.12288
Boecking O, Drescher W (1992) The removal response of Apis mellifera L. colonies to brood in wax and plastic cells after artificial and natural infestation withVarroa jacobsoni Oud. and to freeze-killed brood. Exp & Appl Acarol 16:321-329. https://doi.org/10.1007/bf01218574
Boecking O, Spivak M (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30:141-158. https://doi.org/10.1051/apido:19990205
Borba RS, Klyczek KK, Mogen KL, Spivak M (2015) Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J Exp Biol 218:3689-3699. https://doi.org/10.1242/jeb.127324
Borba RS, Spivak M (2017) Propolis envelope in Apis mellifera colonies supports honey bees against the pathogen, Paenibacillus larvae. Sci Rep 7:11429. https://doi.org/10.1038/s41598-017-11689-w
Brutscher LM, McMenamin AJ, Flenniken ML (2016) The buzz about honey bee viruses. PLoS Pathog 12:e1005757. https://doi.org/10.1371/journal.ppat.1005757
Büchler R, Berg S, Le Conte Y (2010) Breeding for resistance to Varroa destructor in Europe. Apidologie 41:393-408. https://doi.org/10.1051/apido/2010011
Cremer S, Armitage SA, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:R693-702. https://doi.org/10.1016/j.cub.2007.06.008
Cremer S, Sixt M (2009) Analogies in the evolution of individual and social immunity. Philos Trans R Soc Lond B Biol Sci 364:129-142. https://doi.org/10.1098/rstb.2008.0166
Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P (2012) Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl Environ Microbiol 78:981-987. https://doi.org/10.1128/AEM.06537-11
Danka RG, Harris JW, Dodds GE (2016) Selection of VSH-derived “Pol-line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 47:483-490. https://doi.org/10.1007/s13592-015-0413-7
Danka RG, Rinderer TE, Spivak M, Kefuss J (2013) Comments on: “Varroa destructor: research avenues towards sustainable control”. J Apicul Res 52:69-71. https://doi.org/10.3896/Ibra.1.52.2.14
Davis HE, Meconcelli S, Radek R, McMahon DP (2018) Termites shape their collective behavioural response based on stage of infection. Sci Rep 8:14433. https://doi.org/10.1038/s41598-018-32721-7
de Miranda JR, Genersch E (2010) Deformed wing virus. J Invertebr Pathol 103 Suppl 1:S48-61. https://doi.org/10.1016/j.jip.2009.06.012
Donzé G, Guerin PM (1994) Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav Ecol Sociobiol 34:305-319
Donzé G, Guerin PM (1997) Time-activity budgets and space structuring by the different life stages of Varroa jacobsoni in capped brood of the honey bee, Apis mellifera. J Ins Behav10:371-393
Evans JD, Spivak M (2010) Socialized medicine: individual and communal disease barriers in honey bees. J Invertebr Pathol 103 Suppl 1:S62-72. https://doi.org/10.1016/j.jip.2009.06.019
Gerdts J, Dewar RL, Simone Finstrom M, Edwards T, Angove M (2018) Hygienic behaviour selection via freeze-killed honey bee brood not associated with chalkbrood resistance in eastern Australia PLoS One 13:e0203969. https://doi.org/10.1371/journal.pone.0203969
Gilliam M, Taber S, Richardson GV (1983) Hygienic behavior of honey bees in relation to chalkbrood disease. Apidologie 14:29-39. https://doi.org/10.1051/apido:19830103
Gramacho KP, Spivak M (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav Ecol Sociobiol 54:472-479. https://doi.org/10.1007/s00265-003-0643-y
Grozinger C, Flenniken ML (2019) Bee viruses: Ecology, pathogenicity, and impacts. Annu Rev Entomol 64:205-226. https://doi.org/10.1146/annurev-ento-
Guarna M et al. (2016) Expression biomarkers used for the selective breeding of complex polygenic traits. bioRxiv. https://doi.org/10.1101/076174
Guarna MM et al. (2017) Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci Rep 7:8381. https://doi.org/10.1038/s41598-017-08464-2
Harbo JR, Harris JW (1999) Selecting honey bees for resistance to Varroa jacobsoni. Apidologie 30:183-196. https://doi.org/10.1051/apido:19990208
Harbo JR, Harris JW (2005) Suppressed mite reproduction explained by the behaviour of adult bees. J Apicul Res 44:21-23. https://doi.org/10.1080/00218839.2005.11101141
Harbo JR, Harris JW (2009) Responses to Varroa by honey bees with different levels of Varroa Sensitive Hygiene. J Apicul Res 48:156-161. https://doi.org/10.3896/Ibra.1.48.3.02
Harbo JR, Hoopingarner RA (1997) Honey Bees (Hymenoptera: Apidae) in the United States that express resistance to Varroa jacobsoni (Mesostigmata: Varroidae). J Econ Entomol 90:893-898
Harris JW (2007) Bees with Varroa Sensitive Hygiene preferentially remove mite infested pupae aged five days post capping. J Apicul Res 46:134-139. https://doi.org/10.1080/00218839.2007.11101383
Harris JW, Danka RG, Villa JD (2012) Changes in infestation, cell cap condition, and reproductive status of Varroa destructor (Mesostigmata: Varroidae) in brood exposed to honey bees with Varroa sensitive hygiene. Ann Entomol Soc Amer 105:512-518. https://doi.org/10.1603/An11188
Harris JW, Harbo JR (2000) Changes in reproduction of Varroa destructor after honey bee queens were exchanged between resistant and susceptible colonies. Apidologie 31:689-699
Hernandez Lopez J, Schuehly W, Crailsheim K, Riessberger-Galle U (2014) Trans-generational immune priming in honeybees. Proc Biol Sci 281:20140454. https://doi.org/10.1098/rspb.2014.0454
Ibrahim A, Spivak M (2006) The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie 37:31-40. https://doi.org/10.1051/apido:2005052
Invernizzi C, Rivas F, Bettucci L (2011) Resistance to chalkbrood disease in Apis mellifera L. (Hymenoptera: Apidae) colonies with different hygienic behavior. Neotropical Entomol 40:28-34
Jones RL, Rothenbuhler WC (1964) Behaviour genetics of nest cleaning in honey bees. II. Responses of two inbred lines to various amounts of cyanide-killed brood. Anim Behav12:584-588. https://doi.org/10.1016/0003-3472(64)90083-1
Kawasaki T, Kawai T (2019) Discrimination between self and non-self-nucleic acids by the innate immune system. Int Rev Cell Mol Biol 344:1-30. https://doi.org/10.1016/bs.ircmb.2018.08.004
Kirrane MJ, De Guzman LI, Rinderer TE, Frake AM, Wagnitz J, Whelan PM (2011) Asynchronous development of honey bee host and Varroa destructor (Mesostigmata: Varroidae) influences reproductive potential of mites. J Econ Entomol 104:1146-1152. https://doi.org/10.1603/ec11035
Korb J, Fuchs A (2006) Termites and mites – adaptive behavioural responses to infestation. Behaviour 143:891-907
Lachaud JP, Klompen H, Perez-Lachaud G (2016) Macrodinychus mites as parasitoids of invasive ants: an overlooked parasitic association. Sci Rep 6:29995. https://doi.org/10.1038/srep29995
Le Conte Y, Arnold G, Trouiller J, Masson C, Chappe B (1990) Identification of a brood pheromone in honeybees. Naturwissenschaften 77:334-336. https://doi.org/10.1007/bf01138390
Le Conte Y, Hefetz A (2008) Primer pheromones in social Hymenoptera. Annu Rev Entomol 53:523-542. https://doi.org/10.1146/annurev.ento.52.110405.091434
Leclercq G, Francis F, Gengler N, Blacquière T (2018a) Bioassays to quantify hygienic behavior in honey bee (Apis mellifera L.) colonies: A review. J Apicul Res 57:663-673. https://doi.org/10.1080/00218839.2018.1494916
Leclercq G, Blacquière T, Gengler N, Francis F (2018b) Hygienic removal of freeze-killed brood does not predict Varroa-resistance traits in unselected stocks. J Apicul Res 57:292-299. https://doi.org/10.1080/00218839.2018.1426350
Leclercq G, Pannebakker B, Gengler N, Nguyen BK, Francis F (2017) Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): a review. J Apicul Res 56:366-375. https://doi.org/10.1080/00218839.2017.1327938
Leonhardt SD, Menzel F, Nehring V, Schmitt T (2016) Ecology and evolution of communication in social insects. Cell 164:1277-1287. https://doi.org/10.1016/j.cell.2016.01.035
Locke B (2016) Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47:467-482. https://doi.org/10.1007/s13592-015-0412-8
Martin S (1995) Ontogenesis of the mite Varroa jacobsoni Oud. in drone brood of the honeybee Apis mellifera L. under natural conditions. Exp & Appl Acarology 19:199-210. https://doi.org/10.1007/BF00130823
Martin SJ (2001) The role of Varroa and viral pathogens in the collapse of honeybee colonies. J Appl Ecol 38:1082-1093
Martin SJ, Hawkins GP, Brettell LE, Reece N, Correia-Oliveira ME, Allsopp MH (2019) Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie. https://doi.org/10.1007/s13592-019-00721-9
Masterman R, Ross R, Mesce K, Spivak M (2001) Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J Comp Phys A 187:441-452. https://doi.org/10.1007/s003590100216
Masterman R, Smith BH, Spivak M (2000) Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J Ins Behav 13:87-101. https://doi.org/10.1023/A:1007767626594
Maynard Smith J, Harper D (2003) Animal signals. Oxford series in ecology and evolution, 1st edn. Oxford University Press, New York
Medzhitov R, Janeway CAJ (2002) Decoding the patterns of self and nonself by the innate immune system. Science 296:298-300
Mondet F et al. (2020) Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. International J Parasitol 50:433-447. https://doi.org/10.1016/j.ijpara.2020.03.005
Mondet F, Kim SH, de Miranda JR, Beslay D, Le Conte Y, Mercer AR (2016) Specific cues associated with honey bee social defence against Varroa destructor infested brood. Sci Rep 6:25444. https://doi.org/10.1038/srep25444
Mondragon L, Spivak M, Vandame R (2005) A multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie 36:345-358. https://doi.org/10.1051/apido:2005022
Nazzi F, Della Vedova G, D’Agaro M (2004) A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 35:65-70. https://doi.org/10.1051/apido:2003065
Newton DC, Ostasiewski NJJ (1986) A simplified bioassay for behavioral resistance to American foulbrood in honey bees. Amer Bee J126:278-281
Oddie M et al. (2018) Rapid parallel evolution overcomes global honey bee parasite. Sci Rep 8:7704. https://doi.org/10.1038/s41598-018-26001-7
Oddie M et al. (2019) Response to: A small shift in VSH-gene frequency instead of rapid parallel evolution in bees. A comment on Oddie et al. 2018. https://doi.org/10.13140/RG.2.2.14662.22085
Page P et al. (2016) Social apoptosis in honey bee superorganisms. Sci Rep 6:27210. https://doi.org/10.1038/srep27210
Park OW (1937) Testing for resistance to American foulbrood in honeybees. J Econ Entomol 30:504-512. https://doi.org/10.1093/jee/30.3.504
Peng CS, Fang Y, Xu S, Ge L, Nasr ME (1987a) Response of foster Asian honeybee (Apis cerana Fabr.) colonies to the brood of European honeybee (Apis mellifera L.) infested with parasitic mite, Varroa jacobsoni Oudemans. J Invertebr Pathol 49:259-264
Peng YS, Fang YZ, Xu SY, Ge LS (1987b) The resistance mechanism of the Asian honey bee, Apis cerana Fabr, to an ectoparasitic mite, Varroa jacobsoni Oudemans. J Invertebr Pathol 49:54-60. https://doi.org/10.1016/0022-2011(87)90125-X
Perez-Lachaud G, Bartolo-Reyes JC, Quiroa-Montalvan CM, Cruz-Lopez L, Lenoir A, Lachaud JP (2015) How to escape from the host nest: imperfect chemical mimicry in eucharitid parasitoids and exploitation of the ants' hygienic behavior. J Insect Physiol 75:63-72. https://doi.org/10.1016/j.jinsphys.2015.03.003
Perez-Lachaud G, Rocha FH, Valle-Mora J, Henaut Y, Lachaud JP (2019) Fine-tuned intruder discrimination favors ant parasitoidism. PLoS One 14:e0210739. https://doi.org/10.1371/journal.pone.0210739
Pull CD et al. (2018) Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. Elife 7. https://doi.org/10.7554/eLife.32073
Rath W (1999) Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud Apidologie 30:97-110
Raymann K, Moran NA (2018) The role of the gut microbiome in health and disease of adult honey bee workers. Curr Opin Insect Sci 26:97-104. https://doi.org/10.1016/j.cois.2018.02.012
Rinderer TE et al. (2001) Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie 32:381-394
Root AI (1966) The ABC and XYZ of Bee Culture. 33rd Edition. 33th edition edn. The A. I. Root Co., Medina, Ohio
Rose R, Briggs JD (1969) Resistance to American foulbrood in honey bees IX. Effects of honey-bee larval food on the growth and viability of Bacillus larvae. J Invertebr Pathol 13:74-80
Rothenbuhler WC (1964) Behaviour genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease-killed brood. Anim Behav12:578-583. https://doi.org/10.1016/0003-3472(64)90082-x
Schöning C, Gisder S, Geiselhardt S, Kretschmann I, Bienefeld K, Hilker M, Genersch E (2012) Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera J Exp Biol 215:264-271. https://doi.org/10.1242/jeb.062562
Spivak M (1996) Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27:245-260. https://doi.org/10.1051/apido:19960407
Spivak M, Gilliam M (1993) Facultative expression of hygienic behavior of honey bees in relation to disease resistance. J Apicul Res 32:147-157. https://doi.org/10.1080/00218839.1993.11101300
Spivak M, Gilliam M (1998a) Hygienic behaviour of honey bees and its application for control of brood diseases and varroa Part I. Hygienic behaviour and resistance to American foulbrood. Bee World 79:124-134. https://doi.org/10.1080/0005772x.1998.11099394
Spivak M, Gilliam M (1998b) Hygienic behaviour of honey bees and its application for control of brood diseases and varroa - Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee World 79:169-186. https://doi.org/10.1080/0005772x.1998.11099408
Spivak M, Masterman R, Ross R, Mesce KA (2003) Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J Neurobiol 55:341-354. https://doi.org/10.1002/neu.10219
Spivak M, Reuter G (1998) Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 29:291-302
Spivak M, Reuter GS (2001a) Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32:555-565
Spivak M, Reuter GS (2001b) Varroa destructor infestation in untreated honey bee (Hymenoptera Apidae) colonies selected for hygienic behavior. J Econ Entomol 94:326-331
Swanson JA, Torto B, Kells SA, Mesce KA, Tumlinson JH, Spivak M (2009) Odorants that induce hygienic behavior in honeybees: identification of volatile compounds in chalkbrood-infected honeybee larvae. J Chem Ecol 35:1108-1116. https://doi.org/10.1007/s10886-009-9683-8
Toufailia HMA, Amiri E, Scandian L, Kryger P, Ratnieks FL (2014) Towards integrated control of varroa: effect of variation in hygienic behaviour among honey bee colonies on mite population increase and deformed wing virus incidence. J Apicul Res 53:555-562
Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S (2013) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol 23:76-82. https://doi.org/10.1016/j.cub.2012.11.034
van Alphen JJM, Fernhout B (2019) A small shift in VSH-gene frequency instead of rapid parallel evolution in bees. A comment on Oddie et al. 2018 PeerJ Preprints 7. https://doi.org/10.7287/peerj.preprints.27938v2
Vandame R, Morand S, Colin M-E, Belzunces L (2002) Parasitism in the social bee Apis mellifera: Quantifying costs and benefits of behavioral resistance to Varroa destructor mites. Apidologie 33:443-445. https://doi.org/10.1051/apido:2002025
Villa JD, Danka RG, Harris JW (2009) Simplified methods of evaluating colonies for levels of Varroa Sensitive Hygiene (VSH). J Apicul Res 48:162-167. https://doi.org/10.3896/Ibra.1.48.3.03
Villa JD, Danka RG, Harris JW (2016) Selecting honeybees for worker brood that reduces the reproduction of Varroa destructor. Apidologie 47:771-778. https://doi.org/10.1007/s13592-016-0433-y
Villa JD, Danka RG, Harris JW (2017) Repeatability of measurements of removal of mite-infested brood to assess Varroa Sensitive Hygiene. J Apicul Res 56:631-634. https://doi.org/10.1080/00218839.2017.1369707
Villegas AJ, Villa JD (2006) Uncapping of pupal cells by European bees in the United States as responses to Varroa destructor and Galleria mellonella. J Apicul Res 45:203-206. https://doi.org/10.1080/00218839.2006.11101348
Wagoner K, Spivak M, Hefetz A, Reams T, Rueppell O (2019) Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Sci Rep 9:8753. https://doi.org/10.1038/s41598-019-45008-2
Wagoner KM, Spivak M, Rueppell O (2018) Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae). J Econ Entomol 111:2520-2530. https://doi.org/10.1093/jee/toy266
Wilson EO, Durlach NI, Roth LM (1958) Chemical releasers of necrophoric behavior in ants. Psyche 65:108-114
Woodrow AW (1942) Susceptibility of honeybee larvae to individual inoculations with spores of Bacillus larvae. J Econ Entomol 35:892-895. https://doi.org/10.1093/jee/35.6.892
Woodrow A, Holst E (1942) Removal of diseased brood in colonies infected with American foulbrood. Am Bee J 83:22-23
Zhang Y, Han R (2018) A saliva protein of Varroa mites contributes to the toxicity toward Apis cerana and the DWV elevation in A. mellifera. Sci Rep 8:3387. https://doi.org/10.1038/s41598-018-21736-9
Acknowledgments
M. Spivak acknowledges previous funding from the National Science Foundation for studies on hygienic behavior (NSF IBN 9307026, 9722416, and 031991).
Author information
Authors and Affiliations
Contributions
M. Spivak conceived of ideas for the manuscript; R. Danka contributed additional ideas; both authors wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Manuscript editor: James Nieh
Perspectives sur le comportement hygiénique d' Apis mellifera et d'autres insectes sociaux.
immunité sociale / Varroa Sensitive Hygiène / odeurs chimiques / fourmis / termites.
Perspektiven für das Hygieneverhalten bei Apis mellifera und anderen sozialen Insekten.
Soziale Immunität / varroa-sensitive Hygiene / chemische Duftstoffe / Ameisen / Termiten.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on the Apidologie 50 Years
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spivak, M., Danka, R.G. Perspectives on hygienic behavior in Apis mellifera and other social insects. Apidologie 52, 1–16 (2021). https://doi.org/10.1007/s13592-020-00784-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-020-00784-z