Abstract
Euglossine bees are an ecologically important group, which due to their diverse resource needs act as pollinators of many neotropical plants. Male euglossines collect fragrant compounds used in mating displays from diverse sources, including the flowers of orchids and other plants. This aspect of euglossine biology has proven exceptionally useful for studies of euglossine bee populations, because male bees can be readily attracted to fragrance baits deployed in natural habitats. We synthesise the data accumulated over the 50 years since the introduction of euglossine bee baiting inventories and make these data openly available in the EUGCOMM database. By fitting hierarchical joint species distribution models to presence-absence and abundance data, we reveal that the assemblages of bees attracted depend on the baits used in interaction with species-specific fragrance preferences and that bee assemblages are most diverse at sites in landscapes characterised by partial but not complete forest cover. We suggest that these results reflect the diverse resource needs of euglossine bees and are consistent with the hypothesis that male euglossines establish home ranges incorporating multiple habitat types. These results may have important consequences for the design of nature reserves in the tropics, if these iconic pollinators are to be conserved for the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Euglossine bees (‘orchid bees’; Hymenoptera: Apidae: Euglossini) are important pollinators of many neotropical plants (Dressler 1968; Janzen 1971; Williams and Dodson 1972; Armbruster and Webster 1979; Ramírez et al. 2011). Male euglossines collect fragrant compounds from orchids and other floral and non-floral sources to assemble species-specific ‘perfumes’ emitted during courtship displays (Eltz et al. 2005; Zimmermann et al. 2009; Eltz et al. 2015; Weber et al. 2016; Pokorny et al. 2017). This behaviour may play a role in intraspecific mate choice as well as species recognition and speciation (Brand et al. 2015). This characteristic feature of euglossine biology has proven exceptionally useful for studies of euglossine communities, because male bees can be readily attracted to artificial fragrance baits deployed in natural habitats. Fifty years after the introduction of fragrance baits as a census tool (Dodson et al. 1969), our knowledge of male euglossine populations has increased tremendously (Roubik and Hanson 2004). Baiting inventories have now been conducted across most of the range of euglossine bees and have been especially frequent in Brazil over the last 20 years.
The ecological importance of euglossine bees arises in part from their diverse foraging ecology. Both sexes forage for nectar from a large number of plant species (Dressler 1982). Furthermore, female euglossines collect plant resins for use in nest construction and pollen to provision larval nest cells (Dressler 1982; Armbruster 1984; Rocha-Filho et al. 2012; Villanueva-Gutierrez et al. 2013), and male euglossines collect fragrances from diverse sources including rotting wood, fragrant fungi, faeces, and the flowers of orchids and other plants (Whitten et al. 1993; Roubik and Hanson 2004; Ramírez et al. 2011). Because a given habitat may not contain stable supplies of all these resources (nectar, pollen, resin and fragrances), Janzen (1981) suggested that male euglossines have large home ranges incorporating multiple habitat types, within which they would exhibit a nomadic ‘vagabond’ lifestyle and find fragrances, nectar and mates (females) in different habitats (see also Ackerman et al. 1982; Armbruster 1993).
Inventories of euglossine bee assemblages on fragrance baits are valuable not only for understanding the structure of euglossine communities but also as an index of environmental quality and for predicting the reliability of the pollination service provided by the bees. Ackerman (1983) has shown that variation in bee abundance on baits tracks variation in visitation rates of male euglossine bees to orchids. More recently, Opedal et al. (2016, 2017) have shown that the abundance of male euglossine bees on baits predicts the reliability of pollination by female euglossine bees, suggesting that male bee inventories are informative, at least in some cases, about the abundance of females. Thus, data on male euglossines on baits may provide valuable insights into local pollination environments for euglossine bee–pollinated plants, serving as a predictor of both euglossine bee abundance and potential pollination reliability for euglossine-pollinated plants.
The rapid increase in availability of community-level biodiversity data has been paralleled by recent development of statistical methods allowing in-depth analysis of these data. Recently developed joint species distribution models now allow the joint modelling of entire species assemblages, while explicitly considering the multivariate nature of species’ responses to their environment (Warton et al. 2015; Ovaskainen et al. 2017). Current methods also allow assessment of the influence of species’ traits on their response to the environment or other covariates (Abrego et al. 2017).
Here, we synthesise data on euglossine bee assemblages accumulated over the last 50 years. We compiled data on euglossine assemblage composition from published studies conducted using fragrance baits, resulting in the largest openly available database of euglossine bee community samples collected at fragrance baits (EUGCOMM, introduced here), facilitating further studies of assemblage structure, geographic distributions, and effects of landscape structure. To assess the structure of euglossine assemblages and to identify drivers of variation in diversity and abundance, we fitted a series of hierarchical joint species distribution models to subsets of the data. First, we used data from throughout the range of euglossine bees to assess the importance of bait use, species’ bait preferences and sampling effort in determining the assemblage of bees attracted. Second, we used data collected in the Brazilian Atlantic Forest, the most intensively sampled region, to assess environmental drivers of euglossine bee–species richness and abundance. The Atlantic Forest biome has gone through severe deforestation, which has resulted in a highly fragmented landscape. We therefore focus in particular on the effect of landscape structure on euglossine bees.
2 Materials and methods
2.1 Literature survey
To build the database of euglossine bee assemblages (defined here as a sample of a community), we compiled data on bee abundances from published fragrance bait inventories. We started from the lists provided in Ramírez et al. (2015) and Faleiro et al. (2018) and added studies by tracking references within studies and by searching Web of Science and Google Scholar. We included those studies that reported all recorded species, sufficient information about sampling methods and effort, and the number and identities of baits used. Nomenclature follows Nemésio and Rasmussen (2011). We entered species identities as reported in the original studies, except that we corrected obvious typos and inconsistencies. Thus, we chose not to make decisions regarding potential taxonomic problems in the EUGCOMM database.
2.1.1 Sampling effort
We compiled a series of variables describing sampling effort, including sampling method (insect nets, traps, observations, or combinations of these), duration of the study (number of months from first to last sampling day), the number of censuses, the duration of each census (in minutes), the number of baiting stations and/or traps per census, and the number and identities of baits used.
2.1.2 Trait data and bait preferences
We built an additional dataset containing data on phenotypic traits and bait preferences for each species. Bait preferences were quantified as the number of times (i.e. inventories) the species has been collected on a given bait. We included only data for those studies explicitly reporting which bee species were collected on which baits.
2.2 Description of the EUGCOMM database
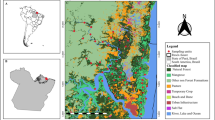
The EUGCOMM database comprises samples of 297 euglossine bee communities collected at 131 study sites distributed throughout most of the range of euglossine bees, from Mexico to Southern Brazil (Figure 1). The 297 assemblages comprise 132,798 individual bees of 172 species representing all five euglossine genera (Euglossa, n = 107 species; Eufriesea, n = 37 species; Eulaema, n = 21 species; Exaerete, n = 6 species; Aglae, n = 1 species). Each study is associated with metadata including the location of the study sites, sampling method and effort, and baits used (Table I). The associated trait database includes a limited amount of trait data (body size, tongue length) and data on the bait preferences of 152 species. The current version of the EUGCOMM database is hosted at GitHub (github.com/oysteiop/eugcomm).
2.3 Joint species distribution models
We analysed several subsets of the data by fitting latent variable joint species distribution models using the Hierarchical Modelling of Species Communities (HMSC) framework of Ovaskainen et al. (2017), implemented in the Hmsc 3.0 R package (Tikhonov et al. 2020).
2.3.1 Effects of bait use and species’ relative bait preferences
Euglossine species differ in the volatiles to which they are attracted, and the assemblage of bees attracted in a study could therefore depend on the set of baits used. To assess these effects, we fitted a HMSC model to presence-absence data for 100 species and 296 samples at 128 sites (Figure 1), excluding very rare species occurring in less than 5 samples. As fixed effects, we included baiting method (net, traps, net + traps, observations), sampling effort, and the use (yes/no) of the seven most commonly used baits (1,8-cineole, eugenol, methyl salicylate, methyl cinnamate, benzyl acetate, vanillin and skatole). As a joint measure of sampling effort, we computed the total duration of sampling as number of censuses × duration of each census in minutes × the number of baiting stations.
To further assess whether the effect of bait use on the probability of attracting individual species depends on their bait preferences, we included relative bait preferences for the same seven baits as species’ traits in the model. This allowed us to estimate what proportion of the bait effects can be explained by species’ relative bait preferences (see Abrego et al. 2017 for details about including traits in the HMSC model). We defined relative bait preference as the number of times a species has been collected on a bait divided by the number of times the species has been collected on any of the baits included in the analysis.
We fitted the model with binomial errors (probit link function) and sampled the posterior distribution with two replicate MCMC chains of 300,000 iterations each, with the first 100,000 discarded as burn-in and a thinning interval of 200, yielding 1000 posterior samples.
2.3.2 Effects of climate and landscape structure on euglossine bee assemblages
To explore environmental predictors of euglossine bee assemblage structure and abundance, we fitted a HMSC model to the data from sites within the Brazilian Atlantic Forest (Mata Atlântica) and neighbouring areas, the most densely sampled region (Figure 1). We excluded very rare species occurring in less than 6 sampling units. This subset of the data comprised 178 sampling units from 72 study areas and a total of 65,008 individuals of 58 species. In this analysis, we pooled Euglossa cordata with Eg. carolina, Euglossa townsendi with Eg. aratingae, and Eulaema cingulata with El. marcii.
We included study site and sampling unit as hierarchical random levels. Study sites were represented by spatially structured latent factors (Ovaskainen et al. 2016), allowing us to model spatial patterns in unmeasured environmental variation. Sampling units within study sites represented either repeated samples over time or samples replicated in space when these were collected along a transect or otherwise intended to represent comprehensive sampling of the study site by incorporating possible within-site heterogeneity (Armbruster 1993).
We extracted altitudes and a set of climate variables (mean annual precipitation, mean annual temperature, precipitation seasonality and temperature seasonality) for each site from WorldClim (Hijmans et al. 2005). We also extracted percentages of land use categories within a 5000-m radius of the sampling site, at 500-m resolution, namely water, urban, pasture, savannah, forest and agriculture (Soares-Filho et al. 2013). From these variables, we computed a measure of land use heterogeneity as the Shannon diversity of the land use categories at each site, i.e. -∑ipi ln pi, where pi is the proportion of land cover belonging to land use category i. The measures of land use heterogeneity and proportion of forest cover were only moderately correlated (r = − 0.34), and we included both in the model because we were interested in the effects of these variables on euglossine bee distributions and abundances. While our main question was how euglossine bees respond to variation in landscape structure, we included the altitude and climate variables to control for differences in bee assemblages along climatic gradients.
To assess whether the four euglossine genera present within the Atlantic Forest (Euglossa, Eufriesea, Eulaema, Exaerete) differ in their responses to any of the covariates included in the model, we included genus as a species ‘trait’ in the model. This allowed us to assess what proportion of variance in species responses to covariates can be explained by euglossine bee genus and to assess whether the genera differ, for example, in their response to landscape structure or climate.
We initially attempted to fit the model with Poisson log-normal errors but experienced poor mixing properties of the MCMC sampling scheme, a known problem in MCMC-based joint species distribution models (Tikhonov et al. 2020). We therefore chose to analyse the data using a ‘hurdle’ approach, where we fitted one model with binomial errors (probit link) to data truncated to presence-absence and a second model with Gaussian errors to log-transformed species abundances conditional on presence (i.e. with all absences set to NA).
We sampled the posterior distributions with two replicate MCMC chains of 150,000 iterations each, with the first 50,000 discarded as burn-in and a thinning interval of 100. We confirmed convergence by computing effective sample sizes and potential scale reduction factors and by visual inspection of posterior trace plots. We evaluated explanatory power for the presence-absence model by computing species-specific coefficients of discrimination (Tjur’s r2) and area under curve (AUC) values and for the abundance model by computing species-specific r2 values on the log scale.
3 Results
3.1 Range-wide diversity patterns
The euglossine bee community samples contained between 1 and 41 species (mean = 13.9 species, median = 12, SD = 7.7) of 1–5 genera (mean = 3.2 genera, median = 3.0, SD = 0.81). Average species richness was consistent across mid-tropical latitudes (between 10°S and ~ 10°N) and declined to the south of 10°S (Figure 2a). We observed only a weak tendency for a similar decline to the north of 10°N, most likely due to very limited sampling. The most common species, Eulaema nigrita, occurred in 284 (85%) of the community samples, while the 27 least common species were sampled only once (Figure 2b).
a Latitudinal patterns of euglossine bee–species richness on fragrance baits. The solid line illustrates a thin-plate spline regression and the dashed lines illustrate the 95% confidence interval of the regression fit. The histogram indicates the distribution of the data. b Rank abundance curve of 172 euglossine bee species across 297 samples.
3.2 Effects of bait use and species’ bait preferences
The model assessing effects of bait use and preferences discriminated well between presences and absences (mean coefficient of discrimination [Tjur r2] = 0.53, mean AUC = 0.98). Of the explained variance, bait use explained 30.0%. Species’ responses to the inclusion of individual baits in the sampling design ranged from positive to negative for all baits, with an overall tendency towards positive effects (Figure 3). The strongest positive marginal effects (i.e. independently of other baits and covariates) were observed for vanillin (mean increase in occurrence probability on probit scale = 1.22) and methyl cinnamate (mean across species = 0.70), while the estimated effect of including 1,8-cineole tended to be negative (mean across species = − 0.89). In turn, species’ bait preferences explained 18.5% of the variance in predicted species occurrences and 26.0% of the variance in species responses to bait use, largely reflecting positive relationships between bait preferences and responses.
Marginal effects of including seven common fragrance baits in euglossine bee baiting inventories on the probability of attracting each of 100 species. Thick lines within boxes indicate medians, boxes extend from the first to third quartile, range bars extend to 1.5× the inter-quartile range, and data points outside this range are shown as open circles.
3.3 Effects of climate and landscape structure on euglossine bee assemblages
The explanatory power of the models fitted to the Atlantic Forest data was reasonably high for both the presence-absence and abundance models, with a mean coefficient of discrimination for presences of 0.48 (range = 0.12–0.89, mean AUC = 0.97) and a mean r2 for abundances conditional on presence of 0.52 (range = 0.02–0.94). Greater proportions of variance in bee distributions and abundances were explained by climate and altitude than by landscape structure (Figure 4, and see Fig. S1 for abundance results).
Variance partitioning for presence-absence of 58 euglossine bee species from the Brazilian Atlantic forest. Colours indicate the contribution of each variable group to the total variance explained by the model for each species. Means in parentheses indicate the mean contribution in percent to the total explained variance. SA, study area, SU, sampling unit.
Euglossine species differed in their response to forest cover, and while some species were most common in forested or non-forested habitats, many species were most common at sites located in landscapes characterised by partial but not complete forest cover, as indicated by negative quadratic effects of forest cover (Figure 5). The four euglossine genera present in the study area also differed in their response to forest cover (Figure 5b, Fig. S2). For example, genus explained 32.1% of the variance among species in the shape of the response to forest cover, as represented by the square term for forest cover.
Effect of forest cover (proportional forest cover within 5 km of the study site) on a total euglossine bee–species richness, b species richness of four euglossine genera and c occurrence probabilities of 58 euglossine bee species on fragrance baits. Predictions were made while holding sampling effort constant, and with sampling method set to ‘Net’. Other covariates were set to vary according to their observed relationship with forest cover.
4 Discussion
Fifty years after the discovery that males of most euglossine bee species can be readily attracted to fragrance baits (Dodson et al. 1969), a substantial amount of data has accumulated on the distribution, diversity, and abundance of euglossine bees. When combined with the powerful analytical tools now available to community ecologists, the product of this long-term effort allows meaningful analyses of the diversity, distributions, and assemblage structure of these ecologically important bees. Furthermore, the standardised and efficient sampling made possible by attracting male euglossines to baits makes the data presented here useful for asking general questions about community structure and species-environment relationships.
4.1 Bait effects on bee attraction reflect species-specific fragrance preferences
Species-specific fragrance preferences are thought to play a central role in the ecology of euglossine bees (Eltz et al. 2005; Zimmermann et al. 2009; Weber et al. 2016; Pokorny et al. 2017). If species differ in the fragrances to which they are attracted, we expect the number of species sampled to increase when more baits are included. The tendency towards positive bait effects detected in our analysis suggests that, unsurprisingly, more species are attracted when additional baits are deployed. This result confirms that the choice of baits used affect the outcome of euglossine baiting inventories by modifying which subset of the local euglossine fauna is attracted. The baits usually deployed in euglossine baiting inventories are important constituents of the floral fragrances of many plants, including species of orchids, Anthurium, and Dalechampia (Ramírez et al. 2011). However, many of these plant species attract only one or a few species of euglossine bees (Armbruster et al. 1992; Ramírez et al. 2011). During their initial experiments with euglossine baiting, Dodson and colleagues (Dodson et al. 1969; Williams and Dodson 1972) noticed that while certain compounds such as eugenol and eucalyptol (1,8-cineole) acted as strong attractants when deployed in pure form, others attracted few or no bees. Furthermore, when mixtures of the pure compounds were deployed, the attractiveness to bees decreased. These observations were interpreted in the light of specificity in chemical communication as a mechanism ensuring reproductive isolation among co-occurring plants through partitioning of pollinator resources. Since these pioneering studies, essentially all euglossine baiting inventories have deployed series of baits rather than mixtures. It is essentially unknown whether mixing that occurs in the environment surrounding the baiting site affects the attractiveness of individual baits, although it seems likely that bees are able to distinguish distinct compounds even when these are deployed near other compounds, as shown for tortricid moths (Potting et al. 1999). Therefore, it seems unlikely that the negative effects observed for specific baits for some species reflect repellent effects, although we encourage further studies assessing potential interactive effects of fragrance composition on attractiveness to male euglossine bees (see also Nemésio 2012). The tendency towards negative effects of including 1,8-cineole is surprising, given that this compound is a strong general attractor. Note, however, that the effects shown in Figure 3 are marginal effects, i.e. effects after controlling for all other variables included in the model, and the effect of including 1,8-cineole in isolation was strongly positive. Finally, while we chose here to analyse only effects of bait use and preferences on the presence of each bee species at baits, the EUGCOMM database would allow further analyses taking advantage of abundance data.
4.2 The euglossine communities of the Brazilian Atlantic Forest
Euglossine bees are thought to be highly susceptible to forest fragmentation and other anthropogenic disturbances. In Southern Costa Rica, for example, euglossines are common within forest fragments but nearly absent from the deforested matrix (Brosi et al. 2008; Brosi 2009). The Brazilian Atlantic Forest (Mata Atlântica) is extremely fragmented, with almost half of the forest cover within less than 100 m from the nearest edge (Ribeiro et al. 2009). Many taxa appear to respond negatively to this forest fragmentation and its drivers (e.g. Chiarello 1999; Uezu and Metzger 2011; Bovendorp et al. 2018), with impacts expected to escalate in the future due to time-lagged responses (Metzger et al. 2009). Our analysis of the euglossine assemblages of the Atlantic Forest revealed that diversity was greatest at sites located in landscapes characterised by high but not complete forest cover. Greater diversity in more heterogeneous environments could arise from a pure additive effect of sampling more diverse habitat types. However, our analysis also revealed that not only species richness but also many individual species exhibited unimodal responses to forest cover. We therefore suggest that the observed greater diversity within heterogeneous landscapes reflects, to some extent, the diverse resource needs of euglossine bees. Indeed, while euglossine bees are famously known as pollinators of certain orchids (hence the common name ‘orchid bees’), it is clear that the ‘perfumes’ assembled by male euglossine bees originate from diverse floral and non-floral sources (Whitten et al. 1993; Pemberton and Wheeler 2006; Ramírez et al. 2011) and that male euglossines visit yet another set of plant species for nectar. The greater diversity and abundance of euglossine bees at sites characterised by mixed land use could therefore arise from the diverse habitat affinities of these plants if, say, some occur in forests, other on forest edges, and yet others in disturbed shrublands. Euglossine bees are known to be exceptionally strong flyers (Janzen 1971; Wikelski et al. 2010; Pokorny et al. 2015), facilitating foraging over large areas. These results suggest that while complete deforestation is without doubt detrimental to euglossine bees and most other wildlife, a certain degree of land use heterogeneity may benefit euglossine bees by allowing multiple resources to be obtained within short distances.
4.3 Possibilities and limitations of the EUGCOMM database
The standardised sampling made possible by attracting male euglossines to fragrance baits suggests that with appropriate controls for sampling effort and method, these data can be readily combined across sampling sites, periods, and studies. This makes such data well suited for testing ecological and biogeographic hypotheses relevant beyond the bees themselves, such as edge and fragmentation effects (e.g. Nemésio and Silveira 2006; Brosi 2009), latitudinal diversity patterns (Abrahamczyk et al. 2014), and effects of euglossine bee abundance on the plants they pollinate (Opedal et al. 2016). The extensive data from the Brazilian Atlantic Forest can be easily combined with other biodiversity data from this well-studied ecoregion. Indeed, the Atlantic Forest has been the subject of extensive biodiversity inventories, with data increasingly made openly available (see the ATLANTIC data paper series; https://github.com/LEEClab/Atlantic_series). The data on euglossine bees presented here adds to openly available data for this region, allowing joint analyses of euglossine bees and other taxa. In contrast to the dense sampling within the Atlantic Forest region, our literature survey also revealed several regions where data are currently scarce, notably central parts of Brazil and northern South America (Venezuela, The Guianas).
Most euglossine bee baiting inventories have been conducted and reported in a way meant to represent the overall communities of euglossine bees at the sampling site, or even in the larger study area. While collections are nearly always made over several days, and often several months, data are typically combined into overall data tables. This dictates the level of analysis to the sampling site level rather than individual sampling events. Thus, with the current data, we can ask questions about variation in euglossine assemblages across sites, but it is hard to study, for example, seasonal variation in bee abundance or bait effects (see Ackerman 1983; Abrahamczyk et al. 2012; Castro et al. 2013). State-of-the-art methods such as the joint species distribution models used here can also be used to assess species associations, i.e. whether some species tend to occur together more or less often than expected from their responses to the environment (Pollock et al. 2014; Ovaskainen et al. 2017). When data from multiple sampling events are pooled, this reduces the power of such analyses because we cannot ascertain whether certain species tend to occur together in the same samples more or less than expected but only whether species tend to occur at the same site. Similarly, data are sometimes collected at multiple sampling stations within apparently homogeneous habitat, yet it has been long known that euglossine assemblages may differ over short distances due to, for example, variation in flowering of preferred species (Armbruster 1993). Without explicit reporting of the raw data, it becomes hard to quantify within-habitat variation with meta-analytical methods. Thus, we strongly encourage making the data from individual samples available in online repositories in future euglossine bee inventories.
4.4 Conclusions: bee assemblages on baits reflect diverse resource needs?
Our initial synthesis of the data accumulated over 50 years of euglossine baiting inventories has revealed that the assemblages of bees attracted to fragrance baits deployed in natural habitats depend on the baits used in interactions with the bait preferences of the local bee fauna. Furthermore, bees were most diverse and abundant at sites located in landscapes characterised by partial but not complete forest cover. These results are consistent with species-specific fragrance preferences of male euglossine bees and suggest that males of at least some euglossine species prefer areas characterised by diverse habitat types, assumingly because these provide a greater range of resources (nectar plants, fragrance plants, nesting sites and resources for females). This view is consistent with Janzen’s hypothesis that male euglossines establish large home ranges including diverse habitat types that jointly provide all necessary resources (Janzen 1981). Therefore, euglossine bees may benefit from consideration of habitat diversity when planning nature reserves and other landscape management in the tropics.
References
Abrahamczyk, S., de Vos, J.M., Sedivy, C., Gottleuber, P., Kessler, M. (2014) A humped latitudinal phylogenetic diversity pattern of orchid bees (Hymenoptera: Apidae: Euglossini) in western Amazonia: assessing the influence of climate and geologic history. Ecography 37, 500–508.
Abrahamczyk, S., Gottleuber, P., Kessler, M. (2012) Seasonal changes in odour preferences by male euglossine bees (Hymenoptera: Apidae) and their ecological implications. Apidologie 43, 212–217.
Abrego, N., Norberg, A., Ovaskainen, O. (2017) Measuring and predicting the influence of traits on the assembly processes of wood-inhabiting fungi. J. Ecol. 105, 1070–1081.
Ackerman, J.D. (1983) Diversity and seasonality of male euglossine bees (Hymenoptera, Apidae) in cental Panama. Ecology 64, 274–283.
Ackerman, J.D., Mesler, M.R., Lu, K.L., Montalvo, A.M. (1982) Food-foraging behavior of male Euglossini (Hymenoptera, Apidae): vagabonds or trapliners? Biotropica 14, 241–248.
Armbruster, W.S. (1984) The role of resin in angiosperm pollination: ecological and chemical considerations. Am. J. Bot. 71, 1149–1160.
Armbruster, W.S. (1993) Within-habitat heterogeneity in baiting samples of male euglossine bees: possible causes and implications. Biotropica 25, 122–128.
Armbruster, W.S., Herzig, A.L., Clausen, T.P. (1992) Pollination of 2 sympatric species of Dalechampia (Euphorbiaceae) in Suriname by male euglossine bees. Am. J. Bot. 79, 1374–1381.
Armbruster, W.S., Webster, G.L. (1979) Pollination of two species of Dalechampia (Euphorbiaceae) in Mexico by euglossine bees. Biotropica 11, 278–283.
Bovendorp, R.S., Brum, F.T., McCleery, R.A., Baiser, B., Loyola, R., Cianciaruso, M.V., Galetti, M. (2018) Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography 42, 23–35.
Brand, P., Ramirez, S.R., Leese, F., Quezada-Euan, J.J., Tollrian, R., Eltz, T. (2015) Rapid evolution of chemosensory receptor genes in a pair of sibling species of orchid bees (Apidae: Euglossini). BMC Evol. Biol. 15, 176.
Brosi, B.J. (2009) The effects of forest fragmentation on euglossine bee communities (Hymenoptera: Apidae: Euglossini). Biol. Conserv. 142, 414–423.
Brosi, B.J., Daily, G.C., Shih, T.M., Oviedo, F., Durán, G. (2008) The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45, 773–783.
Castro, M.M.d.N., Garófalo, C.A., Serrano, J.C., Silva, C.I. (2013) Temporal variation in the abundance of orchid bees (Hymenoptera: Apidae) in a neotropical hygrophilous forest. Sociobiology 60, 405–412.
Chiarello, A.G. (1999) Effects of fragmentation of the Atlantic forest on mammal communities in south-eastern Brazil. Biol. Conserv. 89, 71–82.
Dodson, C.H., Dressler, R.L., Hills, H.G., Adams, R.M., Williams, N.H. (1969) Biologically active compounds in orchid fragrances. Science 164, 1243–1250.
Dressler, R.L. (1968) Pollination by euglossine bees. Evolution 22, 202–210.
Dressler, R.L. (1982) Biology of the orchid bees (Euglossini). Annu. Rev. Ecol. Syst. 13, 373–394.
Eltz, T., Bause, C., Hund, K., Quezada-Euan, J.J.G., Pokorny, T. (2015) Correlates of perfume load in male orchid bees. Chemoecology 25, 193–199.
Eltz, T., Sager, A., Lunau, K. (2005) Juggling with volatiles: exposure of perfumes by displaying male orchid bees. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology 191, 575–581.
Faleiro, F.V., Nemésio, A., Loyola, R. (2018) Climate change likely to reduce orchid bee abundance even in climatic suitable sites. Glob Chang Biol 24, 2272–2283.
Hijmans, R.J., Cameron, S.E., Parra, J.L., Jones, P.G., Jarvis, A. (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25, 1965–1978.
Janzen, D.H. (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205.
Janzen, D.H. (1981) Bee arrival at 2 Costa Rican female Catasetum orchid inflorescenses, and a hypothesis on euglossine population structure. Oikos 36, 177–183.
Metzger, J.P., Martensen, A.C., Dixo, M., Bernacci, L.C., Ribeiro, M.C., Teixeira, A.M.G., Pardini, R. (2009) Time-lag in biological responses to landscape changes in a highly dynamic Atlantic forest region. Biol. Conserv. 142, 1166–1177.
Nemésio, A. (2012) Methodological concerns and challenges in ecological studies with orchid bees (Hymenoptera: Apidae: Euglossina). Bioscience Journal 28, 118–135.
Nemésio, A., Rasmussen, C. (2011) Nomenclatural issues in the orchid bees (Hymenoptera: Apidae: Euglossina) and an updated catalogue. Zootaxa 3006, 1–42.
Nemésio, A., Silveira, F.A. (2006) Edge effects on the orchid-bee fauna (Hymenoptera: Apidae) at a large remnant of atlantic rain forest in southeastern Brazil. Neotrop. Entomol. 35, 313–323.
Opedal, Ø.H., Albertsen, E., Armbruster, W.S., Pérez-Barrales, R., Falahati-Anbaran, M., Pélabon, C. (2016) Evolutionary consequences of ecological factors: pollinator reliability predicts mating-system traits of a perennial plant. Ecol. Lett. 19, 1486–1495.
Opedal, Ø.H., Falahati-Anbaran, M., Albertsen, E., Armbruster, W.S., Pérez-Barrales, R., Stenøien, H.K., Pélabon, C. (2017) Euglossine bees mediate only limited long-distance gene flow in a tropical vine. New Phytol. 213, 1898–1908.
Ovaskainen, O., Roy, D.B., Fox, R., Anderson, B.J. (2016) Uncovering hidden spatial structure in species communities with spatially explicit joint species distribution models. Methods in Ecology and Evolution 7, 428–436.
Ovaskainen, O., Tikhonov, G., Norberg, A., Guillaume Blanchet, F., Duan, L., Dunson, D., Roslin, T., Abrego, N. (2017) How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 20, 561–576.
Pemberton, R.W., Wheeler, G.S. (2006) Orchid bees don't need orchids: evidence from the naturalization of an orchid bee in Florida. Ecology 87, 1995–2001.
Pokorny, T., Loose, D., Dyker, G., Quezada-Euan, J.J.G., Eltz, T. (2015) Dispersal ability of male orchid bees and direct evidence for long-range flights. Apidologie 46, 224–237.
Pokorny, T., Vogler, I., Losch, R., Schlütting, P., Juarez, P., Bissantz, N., Ramírez, S.R., Eltz, T. (2017) Blown by the wind: the ecology of male courtship display behavior in orchid bees. Ecology 98, 1140–1152.
Pollock, L.J., Tingley, R., Morris, W.K., Golding, N., O'Hara, R.B., Parris, K.M., Vesk, P.A., McCarthy, M.A., McPherson, J. (2014) Understanding co-occurrence by modelling species simultaneously with a joint species distribution model (JSDM). Methods in Ecology and Evolution 5, 397–406.
Potting, R.P.J., Lösel, P.M., Scherkenbeck, J. (1999) Spatial discrimination of pheromones and behavioural antagonists by the tortricid moths Cydia pomonella and Adoxophyes orana. J. Comp. Physiol., A 185, 419–425.
Ramírez, S.R., Eltz, T., Fujiwara, M.K., Gerlach, G., Goldman-Huertas, B., Tsutsui, N.D., Pierce, N.E. (2011) Asynchronous diversification in a specialized plant-pollinator mutualism. Science 333, 1742–1746.
Ramírez, S.R., Hernández, C., Link, A., López-Uribe, M.M. (2015) Seasonal cycles, phylogenetic assembly, and functional diversity of orchid bee communities. Ecology and Evolution 5, 1896–1907.
Ribeiro, M.C., Metzger, J.P., Martensen, A.C., Ponzoni, F.J., Hirota, M.M. (2009) The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153.
Rocha-Filho, L.C., Krug, C., Silva, C.I., Garófalo, C.A. (2012) Floral resources used by euglossini bees (Hymenoptera: Apidae) in coastal ecosystems of the Atlantic Forest. Psyche: A Journal of Entomology 2012, 1–13.
Roubik, D.W., Hanson, P.E. (2004) Orchid bees of tropical America. Biology and field guide. INBio, Santo Domingo de Heredia.
Soares-Filho, B.S., Lima, L.S., Hissa, L.B., Costa, W.L.S., Rodrigues, H.O., Ferreira, B.M., Machado, R.F., Campos, A.R., Lima, T.C., Gomes, W.W. (2013) OTIMIZAGRO: Uma plataforma integrada de modelagem de uso e mudanças no uso da terra para o Brasil. IGC/UFMG, Brasilia.
Tikhonov, G., Opedal, Ø.H., Abrego, N., Lehikoinen, A., de Jonge, M.M.J., Oksanen, J. & Ovaskainen, O. 2020. Joint species distribution modelling with the R-package Hmsc. Methods in Ecology and Evolution. https://doi.org/10.1111/2041-210X.13345.
Uezu, A., Metzger, J.P. (2011) Vanishing bird species in the Atlantic Forest: relative importance of landscape configuration, forest structure and species characteristics. Biodivers. Conserv. 20, 3627–3643.
Villanueva-Gutierrez, R., Quezada-Euan, J., Eltz, T. (2013) Pollen diets of two sibling orchid bee species, Euglossa, in Yucatán, southern Mexico. Apidologie 44, 440–446.
Warton, D.I., Blanchet, F.G., O'Hara, R.B., Ovaskainen, O., Taskinen, S., Walker, S.C., Hui, F.K. (2015) So many variables: joint modeling in community ecology. Trends Ecol. Evol. 30, 766–779.
Weber, M.G., Mitko, L., Eltz, T., Ramírez, S.R. (2016) Macroevolution of perfume signalling in orchid bees. Ecol. Lett. 19, 1314–1323.
Whitten, W.M., Young, A.M., Stern, D.L. (1993) Nonfloral sources of chemicals that attract male euglossine bees (Apidae: Euglossini). J. Chem. Ecol. 19, 3017–3027.
Wikelski, M., Moxley, J., Eaton-Mordas, A., Lopez-Uribe, M.M., Holland, R., Moskowitz, D., Roubik, D.W., Kays, R. (2010) Large-range movements of neotropical orchid bees observed via radio telemetry. Plos One 5, e10738 (DOI: 10710.11371/journal.pone.0010738).
Williams, N.H., Dodson, C.H. (1972) Selective attraction of male euglossine bees to orchid floral fragrances and its importance in long distance pollen flow. Evolution 26, 84–95.
Zimmermann, Y., Ramírez, S.R., Eltz, T. (2009) Chemical niche differentiation among sympatric species of orchid bees. Ecology 90, 2994–3008.
Acknowledgements
We thank Scott Armbruster and two anonymous reviewers for comments on the manuscript.
Data accessibility
The EUGCOMM database and associated data and analysis files are available on GitHub (github.com/oysteiop/eugcomm).
Funding
Open access funding provided by Lund University. This work was partly supported by the Research Council of Norway through its Centres of Excellence funding scheme, project number 223257. ØHO was supported by the Finnish Academy (grant nr. 309581 to O. Ovaskainen).
Author information
Authors and Affiliations
Contributions
ØO conceived the ideas and initiated the study. ØO and AAM compiled the literature database. ØO analysed the data with contributions from ELM and wrote the first draft of the manuscript. All authors contributed to revisions.
Corresponding author
Additional information
Manuscript editor: Klaus Hartfelder
Une base de données et une synthèse des collectes d'abeille euglossine prises dans des pièges à odeurs.
Forêt atlantique / Euglossini / Mata Atlântica / abeille à orchidée / interactions plantes-pollinisateurs.
Eine Datenbank und Zusammenstellung von Euglossini-Sammlungen, die in Duftstofffallen gefangen wurden.
Atlantischer Regenwald / Euglossini / Mata Atlantica / Prachtbienen / Pflanzen-Bestäuber-Wechselwirkungen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Opedal, Ø.H., Martins, A.A. & Marjakangas, EL. A database and synthesis of euglossine bee assemblages collected at fragrance baits. Apidologie 51, 519–530 (2020). https://doi.org/10.1007/s13592-020-00739-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-020-00739-4