Abstract
The kinship theory of genomic imprinting argues that conflicting reproductive interests between males and females can lead to epigenetic modifications to the genome, altering gene expression in offspring in a parent-of-origin specific manner. The phenomenon is well documented in mammals and angiosperms, while the evidence for imprinting in social insects is steadily increasing. Workers of the South African honey bee, Apis mellifera capensis (Capensis) produce fatherless female offspring via thelytokous parthenogenesis, whereas queens produce female eggs sexually. We examined differences in reproductive phenotype between thelytokously and sexually derived Capensis workers. Workers with a father had significantly more ovarioles than fatherless workers, suggesting that males may imprint genes to enhance the reproductive success of their worker offspring.
Similar content being viewed by others
1 Introduction
Kin selection theory normally assumes that matrigenes and patrigenes (genes inherited from the mother and father, respectively) are equally expressed in offspring (Queller 2003). Under normal Mendelian inheritance, dominant traits are expressed in offspring regardless of whether they were inherited from the mother or the father. However, differences in the level of investment in offspring by males and females can lead to intersexual conflict, wherein the expression of a gene in one sex has a fitness cost if it is expressed in the other sex. For instance, a father benefits if his offspring can secure more resources from their mother at the expense of half-siblings, with which the father shares no genes. In so doing, he increases his offsprings’ chances of survival, thus maximizing the chance of future spread of his genes throughout the gene pool. Conversely, it is in the mother’s interest to ensure all her offspring are equally provided for, as she is equally related to each. The kinship theory of genomic imprinting (Haig 2000; Haig 2004) argues that such intersexual genomic conflict can manifest as heritable epigenetic modifications to the genome that alter gene expression in offspring in a parent-of-origin specific manner.
The most well-known example of imprinting is the insulin-like growth factor II (IGF-II) gene, which is involved in foetal growth in mammals (Haig and Graham 1991). Only the paternally inherited IGF-II allele is expressed and acts to increase foetal growth (DeChiara et al. 1990; DeChiara et al. 1991). The maternal copy of the allele is epigenetically imprinted, or silenced (Rappolee et al. 1992). Conversely, the maternal copy of the IGF-II receptor (IGF-IIR) is expressed, while the paternal copy is switched off. IGF-IIR appears to act as ‘sink’ for the IGF-II produced by the paternal genes, degrading IGF-II before it can act to increase foetal growth (Haig and Graham 1991). These two differentially expressed genes demonstrate an evolutionary tug-of-war between males and females as each tries to gain the upper hand.
The reproductive biology of the honey bee Apis mellifera provides conditions that are perfect for the evolution of genomic imprinting (Queller 2003; Kronauer 2008; Drewell et al. 2012). Honey bees are haplodiploid. In most honey bee subspecies, diploid female queens and workers arise via sexual reproduction and inherit a set of chromosomes from both their mother and their father. Haploid male drones develop via arrhenotokous parthenogenesis from an unfertilised egg, and thus inherit genetic material from only their mother. Honey bee queens are polyandrous, mating with 10–20 drones (Estoup et al. 1994; Palmer and Oldroyd 2000; Tarpy et al. 2004). Consider a focal worker. Her mother has mated multiple times, and none of the worker’s patrigenes are present in her half sisters. While workers are incapable of mating (Oster and Wilson 1978), under certain circumstances, they can produce haploid male offspring via arrhenotoky (Bourke 1988). Thus, epigenetic modifications to patrigenes that increase the chances of successful worker reproduction should be selected for. In contrast, matrigenes should be modified to prevent worker reproduction, as the queen is more related to her own male offspring than the offspring of daughters (Queller 2003).
South Africa is home to a unique honey bee subspecies, A. m. capensis (hereafter, Capensis). Capensis differs from other subspecies in that unmated workers can produce diploid female offspring without mating via thelytokous parthenogenesis (Onions 1912; Goudie and Oldroyd 2014). Thelytokous reproduction in Capensis has profound effects on the relatedness between colony members. A Capensis worker that reproduces thelytokously is related to her offspring by unity, and thus gains considerable fitness benefits from reproduction if her offspring is raised as a queen. Further, unlike a non-thelytokous honey bee colony, the queen and other workers are predicted to be largely indifferent to worker reproduction, as queens are equally related to their daughters and their thelytokously produced granddaughters. Workers too are equally related to the queen’s offspring and the thelytokous offspring of their sisters (Greeff 1996). As a consequence, worker reproduction occurs at a much higher frequency in Capensis colonies than in arrhenotokous honey bee subspecies, so much so that workers contribute significantly to the production of new queens (Jordan et al. 2008; Allsopp et al. 2010; Holmes et al. 2010; Moritz et al. 2011). The reproductive advantages of thelytoky, particularly queen production, greatly enhance the likelihood of paternal imprinting evolving in this subspecies over other honey bee subspecies (Oldroyd et al. 2014).
The increased reproductive advantage afforded by thelytokous reproduction appears to have enhanced certain reproductive traits in Capensis workers. Although Capensis workers cannot mate, they often possess a spermatheca, the sperm storage organ usually found only in queens (Ruttner 1988). Queenless Capensis workers produce queen-like pheromones that inhibit ovary activation in fellow workers (Moritz et al. 2000). The most significant indicators of increased reproductive capacity in honey bees are ovary size and activation (Makert et al. 2006). Honey bee ovaries are comprised of multiple ovarioles, the tubes within which eggs are formed (Snodgrass 1956). Queens have between 180 and 200 ovarioles per ovary (Snodgrass 1956). While the workers of most honey bee subspecies have between 3 and 5 ovarioles, Capensis usually have between 10 and 20 (Ruttner 1977; Allsopp et al. 2003; Goudie et al. 2012), indicating a shift towards a more ‘queen-like’ physiology.
The discovery that the honey bee possesses a fully functional methylation system (Wang et al. 2006) suggested a mechanism by which honey bees might imprint their genes (Drewell et al. 2012). Subsequently, a number of behavioural, molecular and morphological of studies have provided evidence for paternal imprinting in honey bees. Distinct DNA methylation patterns between honey bee eggs and sperm are suggestive of parent-specific imprinting (Drewell et al. 2014). In reciprocal crosses between Africanised and European honey bee strains, worker offspring with Africanised fathers have significantly more ovarioles and increased patrigenene-biased gene expression than offspring with European fathers (Galbraith et al. 2016). In reciprocal crosses between Capensis and a non-thelytokous subspecies, Apis mellifera scutellata (hereafter Scutellata), worker offspring with a Capensis father are heavier, more likely to possess a spermatheca (Beekman et al. 2012), and have 30% more ovarioles (Oldroyd et al. 2014). Thelytokously- produced diploid Capensis eggs possessing only a maternal genome have fewer hypermethylated genes than sexually- produced eggs with two contributing parental genomes (Remnant et al. 2016).
Here, we compare ovariole number between bi-parental sexually- produced workers and uni-parental thelytokously- produced workers, thereby examining how absence of a paternally-derived genome in a worker affects its reproductive morphology. If males (drones) imprint their genes to increase the reproductive potential of their offspring, then we would expect to find more ovarioles in queen-laid, bi-parental workers relative to the offspring of fatherless workers.
2 Materials and methods
Five virgin Capensis queens were instrumentally inseminated (Harbo 1986), each with the semen of a separate Capensis drone in order to minimise genetic variation among each queen’s worker offspring. This gave us five ‘parental genotypes’: female offspring produced by each queen carried two of only three possible alleles at all loci. After 2 months, we split each colony into two approximately equal halves, one containing the queen and her workers and the other just her workers. The new queenless colonies were checked daily and new queen cells removed. Approximately 10 days after the colonies were split, the queenless workers began laying thelytokously.
Once at least 100 eggs were present in each colony, we cut sections of comb containing eggs from both the queenright and queenless colonies and grafted them into a single brood comb. This composite comb was then placed in a single queenright Capensis colony for the eggs to be reared through to pupation (Oldroyd et al. 2014). However, the nurse colony did not rear eggs from all sources; we were only able to obtain both queen-laid brood and worker-laid brood from just two colonies.
Sixteen days after the eggs were placed in the host colony, the sections of capped brood comb were placed in individual cages in an incubator at 34.5°C. Over the next 3 days, newly emerged workers were collected and frozen. We then dissected all available worker-laid workers and up to 21 queen-laid workers. We removed the left ovary, mounted it in a drop of water on a microscope slide and counted the ovarioles under ×60 magnification.
To ensure that the dissected workers were the progeny of the appropriate queen or her daughter workers, we genotyped each dissected worker and the drone used to inseminate each individual queen at six microsatellite loci in two multiplexes: A08, A14, A79, A88, A113 and B124 (Solignac et al. 2007). Any queen-laid worker that did not possess both the appropriate paternal allele and either one of the two maternal alleles across all six loci was assumed to be the offspring of a non-natal worker and not included in the analysis. Similarly, worker-laid workers that did not possess two copies of either of the two maternal alleles across all six loci were discarded.
We compared ovariole number between the queen-laid and worker-laid offspring, for all colonies and for the two pairs of parental genotypes that produced both worker and queen-laid progeny using separate two-way analyses of variance that included parental genotype and the two-way interaction in the model.
3 Results
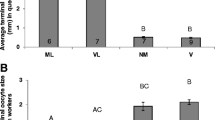
Across all parental genotypes, queen-laid workers had significantly more ovarioles per ovary than asexually produced workers F 1,87 = 6.30, p = 0.014; Figure 1). There was no significant difference in ovariole number among the five parental genotypes (F 1,87 = 2.21, p = 0.07; Figure 1) nor a significant interaction between the two factors (p = 0.92). There were no violations of the assumptions of normality (Shapiro-Wilk test, p > 0.05), or homogeneity of variance (Levene’s test, p = 0.33).
The effects of mode of reproduction and parental genotype on ovariole number in A. m. capensis workers laid by a queen or by workers. Error bars are the standard errors of the means. Numbers above the bars are the sample size. p is the significance of the difference (F test based on the linearly independent pair-wise comparisons).
When considering the two pairs of parental genotypes that produced both queen-laid and worker-laid offspring, queen-laid workers again had more ovarioles than worker-laid workers (F 1,47 = 9.87, p = 0.003; Figure 1). There was no significant difference in ovariole number between the two parental genotypes (F 1,47 = 0.41, p = 0.53) nor a significant interaction between the two factors (F 1,47 = 0.02, p = 0.90). There were no violations of the assumptions of normality (Shapiro-Wilk test, p > 0.05), or homogeneity of variance (Levene’s test, p = 0.89).
Microsatellite genotyping indicated that there were no non-natal worker offspring among any of the queen-laid samples. Eleven workers from queenless colonies were confirmed to be offspring of non-natal workers and were discarded.
4 Discussion
Ovariole number was significantly greater in sexually- produced workers compared with fatherless workers. Our findings therefore support the hypothesis that Capensis fathers epigenetically modify certain genes in order to enhance the reproductive capacities of their female offspring. Our results support previous studies showing strong parent-of-origin effects for reproductive traits in honey bees in general and Capensis in particular (Beekman et al. 2012; Oldroyd et al. 2014; Galbraith et al. 2016).
The proximate mechanisms behind paternal effects on reproductive traits may be direct or indirect. Larval feeding plays a crucial role in determining the reproductive morphology of honey bee workers (Calis et al. 2002). Two compounds produced by worker larvae, methyl linoleate and methyl palmitate, enhance the amount of royal jelly deposited into cells by nurse bees resulting in heavier larvae (Le Conte et al. 1995). Capensis larvae reared in Scutellata colonies elicit more food from their Scutellata hosts, leading to the development of queen-worker intermediates (Calis et al. 2002). Potentially, epigenetically modified patrigenes might enhance the extent of food solicitation behaviour by larvae, providing a mechanism by which reproductive traits are enhanced in adults. Alternatively, imprinted patrigenes may directly modify the rate of programmed cell death that leads to the loss of ovarioles as worker larvae develop (Ronai et al. 2015; Ronai et al. 2016).
Our results are unlikely to arise from differential provisioning of eggs laid by workers and queens. The vast majority of development occurs after the egg hatches into a larva. Our experimental design ensured that larvae had the same feeding opportunities; they were reared together in a single host colony. Queen-laid and worker-laid eggs are indistinguishable in size (Martin et al. 2002) and surface ultrastructure (Katzav-Gozansky et al. 2003). Further, at a population level, approximately half of Capensis queens are the products of thelytokous worker reproduction (Jordan et al. 2008; Allsopp et al. 2010; Holmes et al. 2010; Moritz et al. 2011). Thus, it would appear that queen-laid and worker-laid eggs are provisioned equally, or at least to an adequate threshold, and differences in ovariole number are more likely to be the result of paternal imprinting than physiological provisioning of eggs.
Our study has demonstrated the potential effect of a lack of a paternal genome on the reproductive phenotype in a social insect. Queen-laid Capensis workers have more ovarioles than worker-laid fatherless Capensis workers, indicative of increased reproductive potential. This adds to what is now substantial evidence for genomic imprinting in honey bees.
References
Allsopp M.H., J.N.M. Calis, W.J. Boot (2003) Differential feeding of worker larvae affects caste characters in the Cape honeybee, Apis mellifera capensis. Behav. Ecol. Sociobiol. 54, 555–561.
Allsopp M.H., M. Beekman, R.S. Gloag, B.P. Oldroyd (2010) Maternity of replacement queens in the thelytokous Cape honey bee Apis mellifera capensis. Behav. Ecol. Sociobiol. 64, 567–574.
Beekman M., M.H. Allsopp, M.J. Holmes, J. Lim, L.-A. Noach-Pienaar, T.C. Wossler, B.P. Oldroyd (2012) Racial mixing in South African honeybees: the effects of genotype mixing on reproductive traits of workers. Behav. Ecol. Sociobiol. 66, 897–904.
Bourke A.F.G. (1988) Worker reproduction in the higher eusocial Hymenoptera. Q. Rev. Biol. 63, 291–311.
Calis J.N.M., W.J. Boot, M.H. Allsopp, M. Beekman (2002) Getting more than a fair share: nutrition of worker larvae related to social parasitism in the Cape honey bee Apis mellifera capensis. Apidologie 33, 193–202.
DeChiara T.M., A. Efstratiadis, E.J. Robertsen (1990) A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345, 78–80.
DeChiara T.M., E.J. Robertson, A. Efstratiadis (1991) Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64, 849–859.
Drewell R.A., N. Lo, P.R. Oxley, B.P. Oldroyd (2012) Kin conflict in insect societies: a new epigenetic perspective. Trends Ecol. Evol. 27, 367–373.
Drewell R.A., E.C. Bush, E.J. Remnant, G.T. Wong, S.M. Beeler, J.L. Stringham, J. Lim, B.P. Oldroyd (2014) The dynamic DNA methylation cycle from egg to sperm in the honey bee Apis mellifera. Development 141, 2702–2711.
Estoup A., M. Solignac, J.-M. Cornuet (1994) Precise assessment of the number of patrilines and of genetic relatedness in honey bee colonies. Proc. R. Soc. Lond. B 258, 1–7.
Galbraith D.A., S.D. Kocher, T. Glenn, I. Albert, G.J. Hunt, J.E. Strassmann, D.C. Queller, C.M. Grozinger (2016) Testing the kinship theory of intragenomic conflict in honey bees (Apis mellifera). Proc. Nat. Acad. Sci. USA 113, 1020–1025.
Goudie F., B.P. Oldroyd (2014) Thelytoky in the honey bee. Apidologie 45, 306–326.
Goudie F., M.H. Allsopp, M. Beekman, J. Lim, B.P. Oldroyd (2012) Heritability of worker ovariole number in the Cape honey bee Apis mellifera capensis. Insectes Soc. 59, 351–359.
Greeff J.M. (1996) Effects of thelytokous worker reproduction on kin-selection and conflict in the Cape honeybee, Apis mellifera capensis Phil. Trans. R. Soc. B 351, 617–625.
Haig D. (2000) The kinship theory of genomic imprinting. Annu. Rev. Ecol. Syst. 31, 9–32.
Haig D. (2004) Genomic imprinting and kinship: How good is the evidence? Annu. Rev. Genet. 38, 553–585.
Haig D., C. Graham (1991) Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Cell 64, 1045–1046.
Harbo J.R. (1986) Propagation and instrumental insemination, in: Rinderer T.E. (Ed.), Bee Genetics and Breeding, Academic Press, Orlando, pp. 361–389.
Holmes M.J., B.P. Oldroyd, M.H. Allsopp, J. Lim, T.C. Wossler, M. Beekman (2010) Maternity of emergency queens in the Cape honey bee, Apis mellifera capensis. Mol. Ecol. 19, 2792–2799.
Jordan L.A., M.H. Allsopp, B.P. Oldroyd, T.C. Wossler, M. Beekman (2008) Cheating honeybee workers produce royal offspring., Proc. R. Soc. Lond. Ser. B: Biol. Sci. 275, 345–351.
Katzav-Gozansky T., V. Soroker, J. Kamer, C.M. Schulz, W. Francke, A. Hefetz (2003) Ultrastructural and chemical characterization of egg surface of honeybee worker and queen-laid eggs. Chemoecology 13, 129–134.
Kronauer D.J.C. (2008) Genomic imprinting and kinship in the social Hymenoptera: What are the predictions? J. Theor. Biol. 254, 737–740.
Le Conte Y., L. Sreng, S.H. Poitout (1995) Brood pheromone can modulate the feeding behavior of Apis mellifera workers (Hymenoptera: Apidae). J. Econ. Entomol. 88, 798–804.
Makert G.R., R.J. Paxton, K. Hartfelder (2006) Ovariole number—a predictor of differential reproductive success among worker subfamilies in queenless honeybee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 60, 815–825.
Martin S.J., G.R. Jones, N. Châline, H. Middleton, F.L. Ratnieks (2002) Reassessing the role of the honeybee (Apis mellifera) Dufour’s gland in egg marking. Naturwissenschaften 89, 528–532.
Moritz R.F.A., U.E. Simon, R.M. Crewe (2000) Pheromonal contest between honeybee workers (Apis mellifera capensis). Naturwissenschaften 87, 395–397.
Moritz R.F.A., H.M.G. Lattorff, K.L. Crous, R.H. Hepburn (2011) Social parasitism of queens and workers in the Cape honeybee (Apis mellifera capensis). Behav. Ecol. Sociobiol. 65, 735–740.
Oldroyd B.P., M.H. Allsopp, K.M. Roth, E.J. Remnant, R.A. Drewell, M. Beekman (2014) A parent-of-origin effect on honeybee worker ovary size. Philos. Trans. R. Soc. Lond. B Biol. Sci. 281 20132388.
Onions G.W. (1912) South African ‘fertile worker bees’. Ag. J. Union S. Afric. 1, 720–728.
Oster G.F., E.O. Wilson (1978) Caste and ecology in the social insects, Princeton University Press, Princeton.
Palmer K., B. Oldroyd (2000) Evolution of multiple mating in the genus Apis. Apidologie 31, 235–248.
Queller D.C. (2003) Theory of genomic imprinting conflict in social insects. BMC Evol. Biol. 3:15.
Rappolee D.A., K.S. Sturm, O. Behrendtsen, G.A. Schultz, R.A. Pedersen, Z. Werb (1992) Insulin-like growth factor II acts through an endogenous growth pathway regulated by imprinting in early mouse embryos. Genes Dev. 6, 939–952.
Remnant E.J., A. Ashe, P.E. Young, G. Buchmann, M. Beekman, M.H. Allsopp, C.M. Suter, R.A. Drewell, B.P. Oldroyd (2016) Parent-of-origin effects on genome-wide DNA methylation in the Cape honey bee (Apis mellifera capensis) may be confounded by allele-specific methylation. BMC Genomics 17, 1–14.
Ronai I., D.A. Barton, B.P. Oldroyd, V. Vergoz (2015) Regulation of oogenesis in honey bee workers via programed cell death. J. Insect Physiol. 81, 36–41.
Ronai I., B.P. Oldroyd, D.A. Barton, G. Cabanes, J. Lim, V. Vergoz (2016) Anarchy is a molecular signature of worker sterility in the honey bee. Mol. Biol. Evol. 33, 134–142.
Ruttner F. (1977) The problem of cape bee (Apis mellifera capensis Escholtz)—Parthenogenesis—Size of population—Evolution. Apidologie 8, 281–294.
Ruttner F. (1988) Biogeography and taxonomy of honeybees. Springer-Verlag, Berlin.
Snodgrass R.E. (1956) The anatomy of the honey bee, Comstock, Ithaca.
Solignac M., F. Mougel, D. Vautrin, M. Monnerot, J.-M. Cornuet (2007) A third-generation microsatellite-based linkage map of the honey bee, Apis mellifera, and its comparison with the sequence-based physical map. Genome Biol 8, 1–14.
Tarpy D.R., R. Nielsen, D.I. Nielsen (2004) A scientific note on the revised estimates of paternity frequency in Apis. Insectes Soc. 51, 203–204.
Wang Y., M. Jorda, P.L. Jones, R. Maleszka, X. Ling, H.M. Robertson, C.A. Mizzen, M.A. Peinado, G.E. Robinson (2006) Functional CpG methylation system in a social insect. Science 314, 645–647.
Acknowledgements
We thank Miles Cole-Clarke and Nicholas Smith for help in the field and G. Buchmann for genotyping.
Data accessibility
Raw data are provided in supplementary file ESM 1.
Author information
Authors and Affiliations
Contributions
EMR and BPO designed experiments. RJR, BPO, MB and MAH performed experiments. RJR and BPO analysed data. RJR and BPO wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Funding
This project was funded by the Australian Research Council project DP150100151.
Additional information
Manuscript Editor: Stan Schneider
Effets paternels sur la taille de l’ovaire des ouvrières d’ Apis mellifera capensis
empreinte génétique / conflit génomique / théorie des conflits de parenté
Paternale Effekte auf die Ovariengröße bei Arbeiterinnen von Apis mellifera capensis
Imprinting / genomische Konflikte / Verwandtschaftstheorie
Electronic supplementary material
ESM 1
(XLSX 11 kb).
Rights and permissions
About this article
Cite this article
Reid, R.J., Remnant, E.J., Allsopp, M.H. et al. Paternal effects on Apis mellifera capensis worker ovary size. Apidologie 48, 660–665 (2017). https://doi.org/10.1007/s13592-017-0510-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-017-0510-x