Abstract
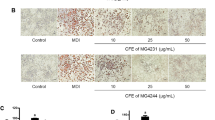
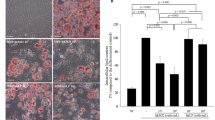
Gut microbial lipopolysaccharides (LPS)-induced inflammatory responses in adipose tissue are associated with the dysfunction of adipocytes, insulin resistance and the development of metabolic syndrome. The aim of this study is to investigate (1) the effects of LPS on the differentiation and inflammatory responses of THP-1 monocytes and OP9 preadipocytes under serum free conditions and (2) the repressive effects of enzyme-digested Colla Corii Asini (CCAD) and fish gelatin (FGD) on LPS-induced inflammatory responses in THP-1 macrophages and OP9 adipocytes. Immunofluorescence and oil red O staining showed that a serum free medium supplied with phorbol 12-myristate 13-acetate (PMA) could induce differentiation and lipid accumulation in THP-1 cells as well as OP9 cells. ELISA showed that LPS significantly increased interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) secretions in PMA-differentiated THP-1 macrophages in a dose-dependent manner. LPS significantly suppressed lipid accumulation and adiponectin secretions, and enhanced IL-6 secretions in OP9 adipocytes. Both CCAD and FGD significantly reduced the levels of both macrophages- and adipocytes-derived inflammatory cytokines and increased the level of OP9-secreted adiponectin. In conclusion, LPS could induce inflammatory responses in both THP-1 and OP9 cells and cause dysfunction of OP9 adipocytes under the serum free conditions. CCAD and FGD can repress LPS-induced inflammatory responses in both THP-1 macrophages and OP9 adipocytes, and increase the secretion of adiponectin in OP9 adipocytes. They could be used as health care supplements for improving metabolic syndrome.







Similar content being viewed by others
References
Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. https://doi.org/10.2337/db07-1403.
Treviño S, Aguilar-Alonso P, Flores Hernandez JA, et al. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 2015;69:421–33. https://doi.org/10.1002/syn.21832.
Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas RC. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–9. https://doi.org/10.1017/S0007114512001213.
Gummesson A, Carlsson LM, Storlien LH, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19:2280–2. https://doi.org/10.1038/oby.2011.251.
Cancello R, Clément K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113:1141–7. https://doi.org/10.1111/j.1471-0528.2006.01004.x.
Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–27. https://doi.org/10.1530/JOE-14-0283.
Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. https://doi.org/10.3389/fphys.2019.01607.
Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and Is, Like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84. https://doi.org/10.1074/jbc.M301977200.
Ye J, Gimble JM. Regulation of stem cell differentiation in adipose tissue by chronic inflammation. Clin Exp Pharm Physiol. 2011;38:872–8. https://doi.org/10.1111/j.1440-1681.2011.05596.x.
Han MS, White A, Perry RJ, et al. Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci USA. 2020;117:2751–60. https://doi.org/10.1073/pnas.1920004117.
Shi C, Zhu L, Chen X, et al. IL-6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J Interf Cytokine Res. 2014;34:342–8. https://doi.org/10.1089/jir.2013.0078.
Clemente-Postigo M, Oliva-Olivera W, Coin-Aragüez L, et al. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. Am J Physiol Endocrinol Metab. 2019;316:E319–32. https://doi.org/10.1152/ajpendo.00277.2018.
Wang D, Ru W, Xu Y, et al. Chemical constituents and bioactivities of Colla corii asini. Drug Discov Ther. 2014;8:201–7. https://doi.org/10.5582/ddt.2014.01038.
Liu T, Zhang P, Ling Y, et al. Protective effect of Colla corii asini against lung injuries induced by intratracheal instillation of artificial fine particles in rats. Int J Mol Sci. 2018;20:55. https://doi.org/10.3390/ijms20010055.
Xiao L, Liao F, Zhou X, Qin Y, Miwa N. Biological effects of traditional Chinese medicine Colla corii asini. New Food Indust. 2019;61:69–72.
Xiao L, Liao F, Ide R, et al. Enzyme-digested Colla Corii Asini (E’jiao) prevents hydrogen peroxide-induced cell death and accelerates amyloid beta clearance in neuronal-like PC12 cells. Neural Regen Res. 2020;15:2270–2. https://doi.org/10.4103/1673-5374.285000.
Xiao L, Liao F, Fan Y, Miwa N. Enzyme-digested Colla Corii Asini (E’jiao) accelerates wound healing and prevents ultraviolet a-induced collagen synthesis decline and wrinkle formation in three-dimensional skin equivalents. Hum Cell. 2020;33:1056–67. https://doi.org/10.1007/s13577-020-00405-y (Published correction appears in Hum Cell. 202134(1):291).
Xiao L, Aoshima H, Saitoh Y, Miwa N. The effect of squalane-dissolved fullerene-C60 on adipogenesis-accompanied oxidative stress and macrophage activation in a preadipocyte-monocyte co-culture system. Biomaterials. 2010;31:5976–85. https://doi.org/10.1016/j.biomaterials.2010.04.032.
Xiao L, Miwa N. Hydrogen nano-bubble water suppresses ros generation, adipogenesis, and interleukin-6 secretion in hydrogen-peroxide- or PMA-stimulated adipocytes and three-dimensional subcutaneous adipose equivalents. Cells. 2021. https://doi.org/10.3390/cells10030626.
Burgess A, Vigneron S, Brioudes E, Labbé JC, Lorca T, Castro A. Loss of human greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA. 2010;107:12564–9. https://doi.org/10.1073/pnas.0914191107.
Xiao L, Mochizuki M, Nakahara T, Miwa N. Hydrogen-generating silica material prevents UVA-ray-induced cellular oxidative stress, cell death, collagen loss and melanogenesis in human cells and 3D skin equivalents. Antioxid (Basel). 2021;10:76. https://doi.org/10.3390/antiox10010076.
Xiao L, Sakagami H, Miwa N. A new method for testing filtration efficiency of mask materials under sneeze-like pressure. In Vivo. 2020;34(3 Suppl):1637–44. https://doi.org/10.21873/invivo.11955.
Antypas H, Libberton B, Melican K. Reducing background cytokine expression in epithelial cells without serum starvation. MethodsX. 2014;1:251–3. https://doi.org/10.1016/j.mex.2014.10.003.
Wolins NE, Quaynor BK, Skinner JR, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–60. https://doi.org/10.1194/jlr.D500037-JLR200.
Castoldi A, Monteiro LB, van Teijlingen BN, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020;11:4107. https://doi.org/10.1038/s41467-020-17881-3.
Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S-261S. https://doi.org/10.3945/ajcn.2009.28449C.
Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47–56. https://doi.org/10.2147/ITT.S73223.
Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: a potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–8. https://doi.org/10.1016/j.cytogfr.2018.01.004.
Longo M, Zatterale F, Naderi J, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358. https://doi.org/10.3390/ijms20092358.
Fiedler T, Salamon A, Adam S, Herzmann N, Taubenheim J, Peters K. Impact of bacteria and bacterial components on osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Exp Cell Res. 2013;319:2883–92. https://doi.org/10.1016/j.yexcr.2013.08.020.
Wang L, Li L, Ran X, et al. Lipopolysaccharides reduce adipogenesis in 3T3-L1 adipocytes through activation of NF-κB pathway and downregulation of AMPK expression. Cardiovasc Toxicol. 2013;13:338–46. https://doi.org/10.1007/s12012-013-9214-2.
Zhao M, Chen X. Effect of lipopolysaccharides on adipogenic potential and premature senescence of adipocyte progenitors. Am J Physiol Endocrinol Metab. 2015;309:E334–44. https://doi.org/10.1152/ajpendo.00601.2014.
Chang CC, Sia KC, Chang JF, et al. Lipopolysaccharide promoted proliferation and adipogenesis of preadipocytes through JAK/STAT and AMPK-regulated cPLA2 expression. Int J Med Sci. 2019;16:167–79. https://doi.org/10.7150/ijms.24068.
Park KI, Lee MR, Oh TW, Kim KY, Ma JY. Antibacterial activity and effects of Colla corii asini on Salmonella typhimurium invasion in vitro and in vivo. BMC Complement Altern Med. 2017;17:520. https://doi.org/10.1186/s12906-017-2020-9.
He F, Wu C, Li P, et al. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed Res Int. 2018;2018:9171905. https://doi.org/10.1155/2018/9171905.
Lee JH, Park E, Jin HJ, et al. Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 2647 macrophages. Food Sci Biotechnol. 2017;26:1371–7. https://doi.org/10.1007/s10068-017-0165-4.
Jayashree B, Bibin YS, Prabhu D, et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203–10. https://doi.org/10.1007/s11010-013-1911-4.
Carnevale R, Nocella C, Petrozza V, et al. Localization of lipopolysaccharide from Escherichia coli into human atherosclerotic plaque. Sci Rep. 2018;8:3598. https://doi.org/10.1038/s41598-018-22076-4.
Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584–9. https://doi.org/10.1086/31509.
Acknowledgements
The present study was supported in part by TaiShan Industrial Experts Program of China No. TSCY20170233 and JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (No. 21H03147 to T.N. and M.M.) and Grant-in-Aid for Young Scientists (B) (No. 21K17029 to M.M.). The authors also appreciate Nathaniel Green’s proofreading.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have reported no potential conflict of interest relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, L., Mochizuki, M., Fan, Y. et al. Enzyme-digested Colla Corii Asini (E’jiao) suppresses lipopolysaccharide-induced inflammatory changes in THP-1 macrophages and OP9 adipocytes. Human Cell 35, 885–895 (2022). https://doi.org/10.1007/s13577-022-00694-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00694-5