Abstract
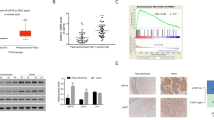
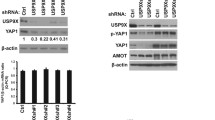
The ectopic expression of ubiquitin-specific peptidase 21 (USP21) is common in different types of cancer. However, its relationship with radio-sensitivity in cervical cancer (CC) remains unclear. In this study, we aimed to uncover the effect of USP21 on CC radio-resistance and its underlying mechanism. Our results showed that the expression of USP21 was markedly increased in CC tissues of radio-resistant patients and CC cells treated with radiation. Besides, knockdown of USP21 restrained the survival fractions, and facilitated apoptosis of CC cells in the absence or presence of radiation. Additionally, USP21 in combination with FOXM1 regulated the stability and ubiquitination of FOXM1. However, FOXM1 reversed the effects of USP21 knockdown on the radio-resistance of CC cells. Furthermore, FOXM1 knockdown activated the Hippo pathway by inhibiting the nuclear translocation of Yes-associated protein 1 (YAP1), and FOXM1 knockdown attenuated the radio-resistance of CC cells via inhibiting the Hippo–YAP1 pathway. USP21 activated the Hippo pathway by mediating FOXM1. Knockdown of USP21 enhanced the radio-sensitivity of CC cells in vivo. In summary, USP21 contributed to the radio-resistance of CC cells via FOXM1/Hippo signaling, and may serve as a promising target for radio-sensitizers in the radiotherapy of CC.








Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Siegel RL, Miller KD. Cancer statistics. CA Cancer J Clin. 2020;70:7–30.
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR. The global burden of women’s cancers: a grand challenge in global health. Lancet (London). 2017;389:847–60.
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (London). 2019;393:169–82.
Prosser SL, O’Regan L, Fry AM. Novel insights into the mechanisms of mitotic spindle assembly by NEK kinases. Mol Cell Oncol. 2016;3:1062952.
Lopata A, Kniss A, Löhr F. Ubiquitination in the ERAD Process. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155369.
Yamano K, Kikuchi R, Kojima W, Hayashida R, Koyano F, Kawawaki J. Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy. J Cell Biol. 2020. https://doi.org/10.1083/jcb.201912144.
Das S, Chandrasekaran AP, Suresh B, Haq S, Kang JH, Lee SJ. Genome-scale screening of deubiquitinase subfamily identifies USP3 as a stabilizer of Cdc25A regulating cell cycle in cancer. Cell Death Differ. 2020;27:3004–20.
Bonacci T, Emanuele MJ. Dissenting degradation: Deubiquitinases in cell cycle and cancer. Semin Cancer Biol. 2020;67:145–58.
Liu J, Kruswick A, Dang H, Tran AD, Kwon SM, Wang XW. Ubiquitin-specific protease 21 stabilizes BRCA2 to control DNA repair and tumor growth. Nat Commun. 2017;8:137.
Chen Y, Zhou B, Chen D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco Targets Ther. 2017;10:681–9.
Arceci A, Bonacci T, Wang X, Stewart K, Damrauer JS, Hoadley KA. FOXM1 deubiquitination by usp21 regulates cell cycle progression and paclitaxel sensitivity in basal-like breast cancer. Cell Rep. 2019;26:3076-3086.e6.
Xu P, Xiao H, Yang Q, Hu R, Jiang L, Bi R. The USP21/YY1/SNHG16 axis contributes to tumor proliferation, migration, and invasion of non-small-cell lung cancer. Exp Mol Med. 2020;52:41–55.
Korver W, Roose J, Clevers H. The winged-helix transcription factor Trident is expressed in cycling cells. Nucleic Acids Res. 1997;25:1715–9.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59.
Liu C, Shi J, Li Q, Li Z, Lou C, Zhao Q. STAT1-mediated inhibition of FOXM1 enhances gemcitabine sensitivity in pancreatic cancer. Clin Sci. 2019;133:645–63.
Xiu G, Sui X, Wang Y, Zhang Z. FOXM1 regulates radiosensitivity of lung cancer cell partly by upregulating KIF20A. Eur J Pharmacol. 2018;833:79–85.
Li T, Ma J, Han X, Jia Y, Yuan H, Shui S. MicroRNA-320 enhances radiosensitivity of glioma through down-regulation of sirtuin type 1 by directly targeting forkhead box protein M1. Transl Oncol. 2018;11:205–12.
Taha Z, Janse van Rensburg HJ, Yang X. The Hippo pathway: immunity and cancer. Cancers. 2018;10:94.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Dey A, Varelas X. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19:480–94.
Zeng Y, Liu Q, Wang Y, Tian C, Yang Q, Zhao Y. CDK5 activates hippo signaling to confer resistance to radiation therapy via upregulating TAZ in lung cancer. Int J Radiat Oncol Biol Phys. 2020;108:758–69.
Zhang S, Zhang X, Sun Q, Zhuang C, Li G, Sun L. LncRNA NR2F2-AS1 promotes tumourigenesis through modulating BMI1 expression by targeting miR-320b in non-small cell lung cancer. J Cell Mol Med. 2019;23:2001–11.
Shibata M, Ham K, Hoque MO. A time for YAP1: tumorigenesis, immunosuppression and targeted therapy. Int J Cancer. 2018;143:2133–44.
Chang Y, Fu XR, Cui M, Li WM, Zhang L, Li X. Activated hippo signal pathway inhibits cell proliferation and promotes apoptosis in NK/T cell lymphoma cells. Cancer Med. 2019;8:3892–904.
Luo J, Yu FX. GPCR-hippo signaling in cancer. Cells. 2019;8:426.
Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–56.
Sun HL, Men JR, Liu HY, Liu MY, Zhang HS. FOXM1 facilitates breast cancer cell stemness and migration in YAP1-dependent manner. Arch Biochem Biophys. 2020;685:108349.
Peng L, Hu Y, Chen D, Jiao S, Sun S. Ubiquitin specific peptidase 21 regulates interleukin-8 expression, stem-cell like property of human renal cell carcinoma. Oncotarget. 2016;7:42007–16.
Mevissen TET, Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu Rev Biochem. 2017;86:159–92.
Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A. Wnt-induced deubiquitination FoxM1 ensures nucleus β-catenin transactivation. EMBO J. 2016;35:668–84.
Yue M, Li S, Yan G, Li C, Kang Z. Paeoniflorin inhibits cell growth and induces cell cycle arrest through inhibition of FoxM1 in colorectal cancer cells. Cell Cycle (Georgetown). 2018;17:240–9.
Nakamura S, Hirano I, Okinaka K, Takemura T, Yokota D, Ono T. The FOXM1 transcriptional factor promotes the proliferation of leukemia cells through modulation of cell cycle progression in acute myeloid leukemia. Carcinogenesis. 2010;31:2012–21.
Varghese V, Magnani L. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9:1505.
Peng WX, Han X, Zhang CL, Ge L, Du FY, Jin J. FoxM1-mediated RFC5 expression promotes temozolomide resistance. Cell Biol Toxicol. 2017;33:527–37.
Nguyen HT, Hong X, Tan S, Chen Q, Chan L, Fivaz M. Viral small T oncoproteins transform cells by alleviating hippo-pathway-mediated inhibition of the YAP proto-oncogene. Cell Rep. 2014;8:707–13.
Kim W, Khan SK, Liu Y, Xu R, Park O, He Y. Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut. 2018;67:1692–703.
Byrne JJ, Soh MS, Chandhok G, Vijayaraghavan T, Teoh JS, Crawford S. Disruption of mitochondrial dynamics affects behaviour and lifespan in Caenorhabditis elegans. Cell Mol Life. 2019;76:1967–85.
Tocci P, Cianfrocca R, Di Castro V, Rosanò L, Sacconi A, Donzelli S. β-arrestin1/YAP/mutant p53 complexes orchestrate the endothelin A receptor signaling in high-grade serous ovarian cancer. Nat Commun. 2019;10:3196.
Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–10.
Fullenkamp CA, Hall SL, Jaber OI, Pakalniskis BL, Savage EC, Savage JM. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget. 2016;7:30094–108.
Bae SJ, Kim M, Kim SH, Kwon YE, Lee JH, Kim J. NEDD4 controls intestinal stem cell homeostasis by regulating the Hippo signalling pathway. Nat Commun. 2015;6:6314.
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72–85.
Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–69.
Hong X, Nguyen HT, Chen Q, Zhang R, Hagman Z, Voorhoeve PM. Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. EMBO J. 2014;33:2447–57.
Nguyen HT, Kugler JM, Loya AC, Cohen SM. USP21 regulates Hippo pathway activity by mediating MARK protein turnover. Oncotarget. 2017;8:64095–105.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
ZLL, XJL, and GXJ made crucial contribution to the conception of this study. ZLL, HZY, and SPW carried out all of the experiments. SLZ prepared the first draft of this manuscript. GXJ agreed to the final design of this work and revised the manuscript critically. XJL performed cell culture and western blotting. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The use of human CC tissues was approved by the Ethics Committee of Yantaishan Hospital (approval No.: 2020016), and the experimental procedures were performed in accordance with the guidelines stipulated in the World Medical Association Declaration of Helsinki. Signed informed consent was obtained from all patients before research initiation. All animal experimental procedures were reviewed and approved by the Ethics Committee of Yantaishan Hospital, and the experimental procedures were performed in accordance with the Guidelines of the National Regulation of China for Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Liu, X., Yu, H. et al. USP21 regulates Hippo signaling to promote radioresistance by deubiquitinating FOXM1 in cervical cancer. Human Cell 35, 333–347 (2022). https://doi.org/10.1007/s13577-021-00650-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00650-9