Abstract
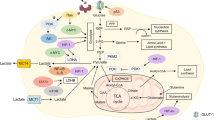
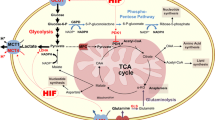
Lactate, as the product of glycolytic metabolism and the substrate of energy metabolism, is an intermediate link between cancer cell and tumor microenvironment metabolism. The exchange of lactate between the two cells via mono-carboxylate transporters (MCTs) is known as the lactate shuttle in cancer. Lactate shuttle is the core of cancer cell metabolic reprogramming between two cells such as aerobic cancer cells and hypoxic cancer cells, tumor cells and stromal cells, cancer cells and vascular endothelial cells. Cancer cells absorb lactate by mono-carboxylate transporter 1 (MCT1) and convert lactate to pyruvate via intracellular lactate dehydrogenase B (LDH-B) to maintain their growth and metabolism. Since lactate shuttle may play a critical role in energy metabolism of cancer cells, components related to lactate shuttle may be a crucial target for tumor antimetabolic therapy. In this review, we describe the lactate shuttle in terms of both substance exchange and regulatory mechanisms in cancer. Meanwhile, we summarize the difference of key proteins of lactate shuttle in common types of cancer.





Similar content being viewed by others
References
Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118(4):691–728. https://doi.org/10.1007/s00421-017-3795-6.
Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9(1):148–63.
Otto Warburg FW, Negelein E. Ueber den stoffwechsel der tumoren. Biochem Z. 1926;152(1):319–44.
Racker E. Bioenergetics and the problem of tumor growth: an understanding of the mechanism of the generation and control of biological energy may shed light on the problem of tumor growth. Am Sci. 1972;60(1):56–63.
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14.
Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4(6):727–32. https://doi.org/10.1242/dmm.007724.
Brooks GA. Lactate: link between glycolytic and oxidative metabolism. Sports Med. 2007;37(4–5):341–3.
Gladden LB. A lactatic perspective on metabolism. Med Sci Sports Exerc. 2008;40(3):477–85. https://doi.org/10.1249/MSS.0b013e31815fa580.
Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558(Pt 1):5–30.
Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30(2):258–64.
Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–42. https://doi.org/10.1172/JCI36843.
Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92(3):329–33. https://doi.org/10.1016/j.radonc.2009.06.025.
Kianercy A, Veltri R, Pienta KJ. Critical transitions in a game theoretic model of tumour metabolism. Interface Focus. 2014;4(4):20140014. https://doi.org/10.1098/rsfs.2014.0014.
Jamali S, Klier M, Ames S, Barros LF, McKenna R, Deitmer JW, et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci Rep. 2015;5:13605. https://doi.org/10.1038/srep13605.
Koiri RK. Lactate as a signaling molecule Journey from dead end product of glycolysis to tumor survival. Front Biosci. 2019;24(2):366–81. https://doi.org/10.2741/4723.
Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10(11):1772–83. https://doi.org/10.4161/cc.10.11.15659.
Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11(6):1108–17. https://doi.org/10.4161/cc.11.6.19530.
Pertega-Gomes N, Vizcaino JR, Attig J, Jurmeister S, Lopes C, Baltazar F. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer. 2014;14:352. https://doi.org/10.1186/1471-2407-14-352.
Becker LM, O’Connell JT, Vo AP, Cain MP, Tampe D, Bizarro L, et al. Epigenetic reprogramming of cancer-associated fibroblasts deregulates glucose metabolism and facilitates progression of breast cancer. Cell Rep. 2020;31(9): 107701. https://doi.org/10.1016/j.celrep.2020.107701.
Garcia-Canaveras JC, Chen L, Rabinowitz JD. The tumor metabolic microenvironment: lessons from lactate. Can Res. 2019;79(13):3155–62. https://doi.org/10.1158/0008-5472.Can-18-3726.
Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18(10):1319–30. https://doi.org/10.2174/138161212799504902.
Perez-Escuredo J, Van Hee VF, Sboarina M, Falces J, Payen VL, Pellerin L, et al. Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta. 2016;1863(10):2481–97. https://doi.org/10.1016/j.bbamcr.2016.03.013.
Miranda-Goncalves V, Bezerra F, Costa-Almeida R, Freitas-Cunha M, Soares R, Martinho O, et al. Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk. Mol Carcinog. 2017;56(12):2630–42. https://doi.org/10.1002/mc.22707.
Payen VL, Mina E, Van Hee VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66. https://doi.org/10.1016/j.molmet.2019.07.006.
Holmes RS, Goldberg E. Computational analyses of mammalian lactate dehydrogenases: human, mouse, opossum and platypus LDHs. Comput Biol Chem. 2009;33(5):379–85. https://doi.org/10.1016/j.compbiolchem.2009.07.006.
Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–92. https://doi.org/10.1172/JCI69741.
Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;118(12):3835–7. https://doi.org/10.1172/JCI37373.
Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272–80. https://doi.org/10.1016/j.canlet.2015.10.027.
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. https://doi.org/10.4161/cc.8.23.10238.
Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A, et al. Understanding the “lethal” drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther. 2010;10(6):537–42. https://doi.org/10.4161/cbt.10.6.13370.
Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9(16):3256–76. https://doi.org/10.4161/cc.9.16.12553.
Benny S, Mishra R, Manojkumar MK, Aneesh TP. From Warburg effect to Reverse Warburg effect; the new horizons of anti-cancer therapy. Med Hypotheses. 2020;144: 110216. https://doi.org/10.1016/j.mehy.2020.110216.
Yu D, Liu C, Guo L. Mitochondrial metabolism and cancer metastasis. Ann Transl Med. 2020;8(14):904. https://doi.org/10.21037/atm.2020.03.42.
Wilde L, Roche M, Domingo-Vidal M, Tanson K, Philp N, Curry J, et al. Metabolic coupling and the Reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198–203. https://doi.org/10.1053/j.seminoncol.2017.10.004.
Lee M, Yoon JH. Metabolic interplay between glycolysis and mitochondrial oxidation: the reverse Warburg effect and its therapeutic implication. World J Biol Chem. 2015;6(3):148–61. https://doi.org/10.4331/wjbc.v6.i3.148.
de Bari L, Atlante A. Including the mitochondrial metabolism of L-lactate in cancer metabolic reprogramming. Cell Mol Life Sci. 2018;75(15):2763–76. https://doi.org/10.1007/s00018-018-2831-y.
Ippolito L, Morandi A, Taddei ML, Parri M, Comito G, Iscaro A, et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene. 2019;38(27):5339–55. https://doi.org/10.1038/s41388-019-0805-7.
Baltazar F, Afonso J, Costa M, Granja S. Lactate beyond a waste metabolite: metabolic affairs and signaling in malignancy. Front Oncol. 2020;10:231. https://doi.org/10.3389/fonc.2020.00231.
Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE. 2012;7(3): e33418. https://doi.org/10.1371/journal.pone.0033418.
Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71(7):2550–60. https://doi.org/10.1158/0008-5472.CAN-10-2828.
Goodwin ML, Pennington Z, Westbroek EM, Cottrill E, Ahmed AK, Sciubba DM. Lactate and cancer: a “lactatic” perspective on spinal tumor metabolism (part 1). Ann Transl Med. 2019;7(10):220. https://doi.org/10.21037/atm.2019.02.32.
Gorska-Ponikowska M, Kuban-Jankowska A, Daca A, Nussberger S. 2-Methoxyestradiol reverses the pro-carcinogenic effect of L-Lactate in Osteosarcoma 143B cells. Cancer Genomics Proteomics. 2017;14(6):483–93. https://doi.org/10.21873/cgp.20058.
Bonuccelli G, Avnet S, Grisendi G, Salerno M, Granchi D, Dominici M, et al. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget. 2014;5(17):7575–88. https://doi.org/10.18632/oncotarget.2243.
Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, et al. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278(33):31277–85. https://doi.org/10.1074/jbc.M300763200.
Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271(49):31372–8. https://doi.org/10.1074/jbc.271.49.31372.
Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25(57):7482–91. https://doi.org/10.1038/sj.onc.1210055.
Chafey P, Finzi L, Boisgard R, Cauzac M, Clary G, Broussard C, et al. Proteomic analysis of beta-catenin activation in mouse liver by DIGE analysis identifies glucose metabolism as a new target of the Wnt pathway. Proteomics. 2009;9(15):3889–900. https://doi.org/10.1002/pmic.200800609.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. https://doi.org/10.1016/j.devcel.2009.06.016.
Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25(57):7492–504. https://doi.org/10.1038/sj.onc.1210056.
Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33(13):1454–73. https://doi.org/10.15252/embj.201488598.
Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24(13):5923–36. https://doi.org/10.1128/MCB.24.13.5923-5936.2004.
Hyunsuk Shim CD, Brian C, Lewis,Chyi-Sun Wu, Gerard Dang, Richard A. Jungmann, Riccardo Dalla-Favera, and Chi V, Dang. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Biochemistry. 1997;94(66): 6658–63.
Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275(29):21797–800. https://doi.org/10.1074/jbc.C000023200.
Toshiya Atsumi JC, Christine Metz, Lin Leng, Seamas Donnelly, Zenji Makita, Robert Mitchell, and Richard Bucala. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62: 5881–7.
Li H, Jogl G. Structural and biochemical studies of TIGAR (TP53-induced glycolysis and apoptosis regulator). J Biol Chem. 2009;284(3):1748–54. https://doi.org/10.1074/jbc.M807821200.
Liu Z, Wu Y, Zhang Y, Yuan M, Li X, Gao J, et al. TIGAR promotes tumorigenesis and protects tumor cells from oxidative and metabolic stresses in gastric cancer. Front Oncol. 2019;9:1258. https://doi.org/10.3389/fonc.2019.01258.
Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, et al. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem. 2002;277(8):6183–7. https://doi.org/10.1074/jbc.M110978200.
Ramljak S, Herlyn H, Zerr I. Cellular Prion Protein (PrP(c)) and Hypoxia: true to each other in good times and in bad, in sickness, and in health. Front Cell Neurosci. 2016;10:292. https://doi.org/10.3389/fncel.2016.00292.
Burns JS, Manda G. Metabolic pathways of the Warburg effect in health and disease: perspectives of choice, chain or chance. Int J Mol Sci. 2017;18(12):2755. https://doi.org/10.3390/ijms18122755.
Wanka C, Brucker DP, Bahr O, Ronellenfitsch M, Weller M, Steinbach JP, et al. Synthesis of cytochrome C oxidase 2: a p53-dependent metabolic regulator that promotes respiratory function and protects glioma and colon cancer cells from hypoxia-induced cell death. Oncogene. 2012;31(33):3764–76. https://doi.org/10.1038/onc.2011.530.
Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36(10):1302–15. https://doi.org/10.15252/embj.201696151.
Kim GW, Lee DH, Jeon YH, Yoo J, Kim SY, Lee SW, et al. Glutamine synthetase as a therapeutic target for cancer treatment. Int J Mol Sci. 2021;22(4):1701. https://doi.org/10.3390/ijms22041701.
Otto AM, Hintermair J, Janzon C. NADH-linked metabolic plasticity of MCF-7 breast cancer cells surviving in a nutrient-deprived microenvironment. J Cell Biochem. 2015;116(5):822–35. https://doi.org/10.1002/jcb.25038.
Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–40. https://doi.org/10.1158/0008-5472.CAN-12-1949.
Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14(12):2361–71. https://doi.org/10.1089/ars.2010.3727.
Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal. 2012;16(11):1264–84. https://doi.org/10.1089/ars.2011.4243.
Nocquet L, Juin PP, Souaze F. Mitochondria at center of exchanges between cancer cells and cancer-associated fibroblasts during tumor progression. Cancer (Basel). 2020;12(10):3017. https://doi.org/10.3390/cancers12103017.
Morandi A, Chiarugi P. Metabolic implication of tumor:stroma crosstalk in breast cancer. J Mol Med (Berl). 2014;92(2):117–26. https://doi.org/10.1007/s00109-014-1124-7.
Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27(19):2072–85. https://doi.org/10.1101/gad.227439.113.
Frederic Bost LK. The metabolic modulator PGC-1α in cancer. Am J Cancer Res. 2019;9(2):198–211.
Taddei ML, Cavallini L, Comito G, Giannoni E, Folini M, Marini A, et al. Senescent stroma promotes prostate cancer progression: the role of miR-210. Mol Oncol. 2014;8(8):1729–46. https://doi.org/10.1016/j.molonc.2014.07.009.
Chandler C, Liu T, Buckanovich R, Coffman LG. The double edge sword of fibrosis in cancer. Transl Res. 2019;209:55–67. https://doi.org/10.1016/j.trsl.2019.02.006.
Pertega-Gomes N, Felisbino S, Massie CE, Vizcaino JR, Coelho R, Sandi C, et al. A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: a role for monocarboxylate transporters as metabolic targets for therapy. J Pathol. 2015;236(4):517–30. https://doi.org/10.1002/path.4547.
Ruan Y, Zeng F, Cheng Z, Zhao X, Fu P, Chen H. High expression of monocarboxylate transporter 4 predicts poor prognosis in patients with lung adenocarcinoma. Oncol Lett. 2017;14(5):5727–34. https://doi.org/10.3892/ol.2017.6964.
Giatromanolaki A, Koukourakis MI, Koutsopoulos A, Mendrinos S, Sivridis E. The metabolic interactions between tumor cells and tumor-associated stroma (TAS) in prostatic cancer. Cancer Biol Ther. 2012;13(13):1284–9. https://doi.org/10.4161/cbt.21785.
Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2011;43(5):255–64. https://doi.org/10.1152/physiolgenomics.00177.2010.
Koukourakis MI, Giatromanolaki A, Bougioukas G, Sivridis E. Lung cancer: a comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biol Ther. 2007;6(9):1476–9. https://doi.org/10.4161/cbt.6.9.4635.
Alves VA, Pinheiro C, Morais-Santos F, Felipe-Silva A, Longatto-Filho A, Baltazar F. Characterization of monocarboxylate transporter activity in hepatocellular carcinoma. World J Gastroenterol. 2014;20(33):11780–7. https://doi.org/10.3748/wjg.v20.i33.11780.
Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44(1):127–39. https://doi.org/10.1007/s10863-012-9428-1.
Nelma Pértega-Gomes JRV, Miranda-Gonçalves V, Pinheiro C, Silva J, Pereira H, Monteiro P, Henrique RM, Reis RM, Lopes C, Baltazar F. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer. 2011;11:3–12.
Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56(7):860–7. https://doi.org/10.1111/j.1365-2559.2010.03560.x.
Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, et al. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9(10):1960–71. https://doi.org/10.4161/cc.9.10.11601.
Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol. 2010;2010: 427694. https://doi.org/10.1155/2010/427694.
Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36(Pt A):143–51. https://doi.org/10.1016/j.ijsu.2016.10.033.
Liu X, Yang Z, Chen Z, Chen R, Zhao D, Zhou Y, et al. Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human gastric cancer cells. Oncol Rep. 2015;33(1):157–62. https://doi.org/10.3892/or.2014.3600.
Pinheiro C, Longatto-Filho A, Simoes K, Jacob CE, Bresciani CJ, Zilberstein B, et al. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. Eur J Cancer. 2009;45(13):2418–24. https://doi.org/10.1016/j.ejca.2009.06.018.
Pinheiro C, Longatto-Filho A, Ferreira L, Pereira SM, Etlinger D, Moreira MA, et al. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int J Gynecol Pathol. 2008;27(4):568–74. https://doi.org/10.1097/PGP.0b013e31817b5b40.
Pinheiro C, Longatto-Filho A, Pereira SM, Etlinger D, Moreira MA, Jube LF, et al. Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma. Dis Markers. 2009;26(3):97–103. https://doi.org/10.3233/DMA-2009-0596.
Pinheiro C, Garcia EA, Morais-Santos F, Moreira MA, Almeida FM, Jube LF, et al. Reprogramming energy metabolism and inducing angiogenesis: co-expression of monocarboxylate transporters with VEGF family members in cervical adenocarcinomas. BMC Cancer. 2015;15:835. https://doi.org/10.1186/s12885-015-1842-4.
Subramanian N, Krishnan HM, Venkatachalam P, Kamatchi PAC. A study of Lactate Dehydrogenase (LDH) Isoenzyme is a biochemical tumour marker in cervical carcinoma patients. Int J Hum Genet. 2017;9(1):5–12. https://doi.org/10.1080/09723757.2009.11886055.
Afonso J, Santos LL, Miranda-Goncalves V, Morais A, Amaro T, Longatto-Filho A, et al. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol Carcinog. 2015;54(11):1451–66. https://doi.org/10.1002/mc.22222.
Jiang F, Ma S, Xue Y, Hou J, Zhang Y. LDH-A promotes malignant progression via activation of epithelial-to-mesenchymal transition and conferring stemness in muscle-invasive bladder cancer. Biochem Biophys Res Commun. 2016;469(4):985–92. https://doi.org/10.1016/j.bbrc.2015.12.078.
Choi JW, Kim Y, Lee JH, Kim YS. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology. 2014;84(1):245 e9-245 e15. https://doi.org/10.1016/j.urology.2014.03.031.
Martins SF, Amorim R, Viana-Pereira M, Pinheiro C, Costa RF, Silva P, et al. Significance of glycolytic metabolism-related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer. 2016;16:535. https://doi.org/10.1186/s12885-016-2566-9.
Roseweir AK, Clark J, McSorley ST, vanWyk HC, Quinn JA, Horgan PG, et al. The association between markers of tumour cell metabolism, the tumour microenvironment and outcomes in patients with colorectal cancer. Int J Cancer. 2019;144(9):2320–9. https://doi.org/10.1002/ijc.32045.
Miranda-Goncalves V, Honavar M, Pinheiro C, Martinho O, Pires MM, Pinheiro C, et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 2013;15(2):172–88. https://doi.org/10.1093/neuonc/nos298.
Koukourakis M, Tsolou A, Pouliliou S, Lamprou I, Papadopoulou M, Ilemosoglou M, et al. Blocking LDHA glycolytic pathway sensitizes glioblastoma cells to radiation and temozolomide. Biochem Biophys Res Commun. 2017;491(4):932–8. https://doi.org/10.1016/j.bbrc.2017.07.138.
Noble RA, Bell N, Blair H, Sikka A, Thomas H, Phillips N, et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2017;102(7):1247–57. https://doi.org/10.3324/haematol.2016.163030.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. https://doi.org/10.1016/j.ccr.2012.02.022.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–90. https://doi.org/10.3390/cancers6031670.
Kes MMG, Van den Bossche J, Griffioen AW, Huijbers EJM. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. Biochim Biophys Acta Rev Cancer. 2020;1874(2): 188427. https://doi.org/10.1016/j.bbcan.2020.188427.
Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280(51):41928–39. https://doi.org/10.1074/jbc.M508718200.
Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia Y, et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2alpha-mediated tumor progression. J Clin Invest. 2019;129(2):631–46. https://doi.org/10.1172/JCI123027.
Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114(3):580–5. https://doi.org/10.1073/pnas.1614035114.
Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17(4):428–38. https://doi.org/10.1080/15384101.2018.1444305.
Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–9. https://doi.org/10.1182/blood-2006-07-035972.
Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131(3):633–40. https://doi.org/10.1002/ijc.26410.
Ping W, Senyan H, Li G, Yan C, Long L. Increased lactate in gastric cancer tumor-infiltrating lymphocytes is related to impaired T cell function due to miR-34a deregulated lactate dehydrogenase A. Cell Physiol Biochem. 2018;49(2):828–36. https://doi.org/10.1159/000493110.
Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–71. https://doi.org/10.1016/j.cmet.2016.08.011.
Scott KE, Cleveland JL. Lactate Wreaks Havoc on tumor-infiltrating T and NK cells. Cell Metab. 2016;24(5):649–50. https://doi.org/10.1016/j.cmet.2016.10.015.
Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res. 2019;7(2):335–46. https://doi.org/10.1158/2326-6066.CIR-18-0481.
Husain Z, Huang YN, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95. https://doi.org/10.4049/jimmunol.1202702.
Dai H, Xu H, Wang S, Ma J. Connections between metabolism and epigenetic modification in MDSCs. Int J Mol Sci. 2020;21(19):7356. https://doi.org/10.3390/ijms21197356.
Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology. Oncoimmunology. 2013;2(11): e26383. https://doi.org/10.4161/onci.26383.
Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–21. https://doi.org/10.1182/blood-2005-05-1795.
Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206: 107451. https://doi.org/10.1016/j.pharmthera.2019.107451.
Lundo K, Trauelsen M, Pedersen SF, Schwartz TW. Why Warburg works: lactate controls immune evasion through GPR81. Cell Metab. 2020;31(4):666–8. https://doi.org/10.1016/j.cmet.2020.03.001.
Puri S, Juvale K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: a review with structure-activity relationship insights. Eur J Med Chem. 2020;199: 112393. https://doi.org/10.1016/j.ejmech.2020.112393.
Becker HM. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer. 2020;122(2):157–67. https://doi.org/10.1038/s41416-019-0642-z.
Van Wilpe S, Koornstra R, Den Brok M, De Groot JW, Blank C, De Vries J, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology. 2020;9(1):1731942. https://doi.org/10.1080/2162402X.2020.1731942.
Zhang SL, He Y, Tam KY. Targeting cancer metabolism to develop human lactate dehydrogenase (hLDH)5 inhibitors. Drug Discov Today. 2018;23(7):1407–15. https://doi.org/10.1016/j.drudis.2018.05.014.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. https://doi.org/10.1016/j.cmet.2015.12.006.
Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021. https://doi.org/10.1038/s41586-021-03442-1.
Acknowledgements
This work was supported by Shandong Province Natural Science Foundation for Cancer Prevention Governance Joint Fund (No. ZR2019LZL005) and Natural Science Foundation of Shandong Province (No. ZR2017MH028) of P. R. China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, H., Ni, Y. et al. Lactate shuttle: from substance exchange to regulatory mechanism. Human Cell 35, 1–14 (2022). https://doi.org/10.1007/s13577-021-00622-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00622-z