Abstract
Over the past decades, stem cell therapy has been investigated as a promising approach towards various diseases, including neurodegenerative disorders. Stem cells show the capability to differentiate into neuronal progenitor cells in vitro. In the present study, the differentiation potential of human-induced pluripotent stem cells (hiPSCs) into neural lineages was examined under the efficient induction media containing forskolin and 3-isobutyl-1-methyl-xanthine (IBMX) in the presence of nisin (Ni), non-essential amino acids (NEAA) and combination of those (NEAA-Ni) in vitro. The optimum concentrations of these factors were obtained by MTT assay and acridine orange (AO) staining. The effect of Ni and NEAA on the expression rate of neural-specific markers including NSE, MAP2, and ß-tubulin III was studied via immunocytochemistry (ICC) and real-time RT-PCR analyses. Our results indicated that the induction medium containing Ni or NEAA increased the gene and protein expression of NSE, MAP2, and β-tubulin III on the 14th differentiation day. On the other hand, NEAA-Ni showed a less-differentiated hiPSCs compared to Ni and NEAA alone. In conclusion, the obtained results illustrated that Ni and NEAA could be applied as effective factors for neural differentiation of hiPSCs in the future.
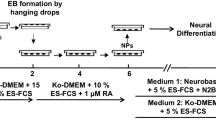
Graphic abstract





Similar content being viewed by others
Abbreviations
- AO:
-
Acridine orange
- AA:
-
Amino acids
- cAMP:
-
Cyclic adenosine monophosphate
- ESCs:
-
Embryonic stem cells
- EB:
-
Ethidium Bromide
- hiPSCs:
-
Human induced pluripotent stem cells
- IBMX:
-
3-Isobutyl-1-methyl-xanthine
- MSCs:
-
Mesenchymal stem cells
- MAP-2:
-
Microtubule-associated protein-2
- NSE:
-
Neuron-specific enolase
- Ni:
-
Nisin
- NEAA-Ni:
-
Ni in combination with NEAA
- NEAA:
-
Non-essential amino acids
- RNA:
-
Ribonucleic acids
References
Lu P. Stem cell transplantation for spinal cord injury repair. Prog Brain Res. 2017;231:1–32.
Yuan T, Liu Q, Kang J, Gao H, et al. High-dose neural stem/progenitor cell transplantation increases engraftment and neuronal distribution and promotes functional recovery in rats after acutely severe spinal cord injury. Stem Cells Int. 2019;2019:9807978.
Silvestro S, Bramanti P, Trubiani O, Mazzon E. Stem cells therapy for spinal cord injury: an overview of clinical trials. Int J Mol Sci. 2020;21(2):659.
Pittenger MF, Discher DE, Péault BM, Phinney DG, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4(1):22.
Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, et al. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14(2):227–37.
Wang YH, Guo YC, Wang DR, Liu JY, et al. Adipose stem cell-based clinical strategy for neural regeneration: a review of current opinion. Stem Cells Int. 2019;2019:8502370.
Sugaya K, Vaidya M. Stem cell therapies for neurodegenerative diseases. Adv Exp Med Biol. 2018;1056:61–84.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–9.
Ghaedi M, Niklason LE. Human pluripotent stem cells (iPSC) generation, culture, and differentiation to lung progenitor cells. Methods Mol Biol. 2019;1576:55–92.
Nakao S, Ihara D, Hasegawa K, Kawamura T. Applications for induced pluripotent stem cells in disease modelling and drug development for heart diseases. Eur Cardiol. 2020;15:1–10.
Castro-Viñuelas R, Sanjurjo-Rodríguez C, Piñeiro-Ramil M, Hermida-Gómez T, et al. Generation and characterization of human induced pluripotent stem cells (iPSCs) from hand osteoarthritis patient-derived fibroblasts. Sci Rep. 2020;10(1):4272.
Genova E, Cavion F, Lucafò M, Leo L, et al. Induced pluripotent stem cells for therapy personalization in pediatric patients: Focus on drug-induced adverse events. World J Stem Cells. 2019;11(12):1020–44.
Zhang H, Shao X, Peng Y, Teng Y, et al. A novel machine learning based approach for iPS progenitor cell identification. PLoS Comput Biol. 2019;15(12):e1007351.
Bertucci TB, Dai G. Biomaterial engineering for controlling pluripotent stem cell fate. Stem Cells Int. 2018;2018:9068203.
Zhang FQ, Jiang JL, Zhang JT, Niu H, et al. Current status and future prospects of stem cell therapy in Alzheimer’s disease. Neural Regen Res. 2020;15(2):242–50.
Oh Y. Patient-specific pluripotent stem cell-based Parkinson’s disease models showing endogenous alpha-synuclein aggregation. BMB Rep. 2019;52(6):349–59.
Rindt H, Tom CM, Lorson CL, Mattis VB. Optimization of trans-Splicing for Huntington’s Disease RNA Therapy. Front Neurosci. 2017;11:544.
Csobonyeiova M, Polak S, Zamborsky R, Danisovic L. Recent progress in the regeneration of spinal cord injuries by induced pluripotent stem cells. Int J Mol Sci. 2019;20(15):3838.
Verpelli C, Carlessi L, Bechi G, Fusar PE, et al. Comparative neuronal differentiation of self-renewing neural progenitor cell lines obtained from human induced pluripotent stem cells. Front Cell Neurosci. 2013;7:175.
Wu YY, Chiu FL, Yeh CS, Kuo HC. Opportunities and challenges for the use of induced pluripotent stem cells in modelling neurodegenerative disease. Open Biol. 2019;9(1):180177.
Denham M, Dottori M. Neural differentiation of induced pluripotent stem cells. Methods Mol Biol. 2011;793:99–110.
Salimi A, Nadri S, Ghollasi M, Khajeh K, et al. Comparison of different protocols for neural differentiation of human induced pluripotent stem cells. Mol Biol Rep. 2014;41(3):1713–21.
Thompson R, Casali C, Chan C. Forskolin and IBMX induce neural transdifferentiation of MSCs through downregulation of the NRSF. Sci Rep. 2019;9(1):2969.
Shahbazi A, Safa M, Alikarami F, Kargozar S, et al. Rapid induction of neural differentiation in human umbilical cord matrix mesenchymal stem cells by cAMP-elevating agents. Int J Mol Cell Med. 2016;5(3):167–77.
Małaczewska J, Kaczorek-Łukowska E, Wójcik R, Siwicki AK. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet Res. 2019;15(1):318.
Garcia-Gutierrez E, O’Connor PM, Saalbach G, Walsh CJ, et al. First evidence of production of the lantibiotic nisin P. Sci Rep. 2020;10(1):3738.
Ge X, Teng K, Wang J, Zhao F, et al. Ligand determinants of nisin for its induction activity. J Dairy Sci. 2016;99(7):5022–31.
Kamarajan P, Hayami T, Matte B, Liu Y, et al. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS ONE. 2015;10(7):e0131008.
Zainodini N, Hassanshahi G, Hajizadeh M, Khanamani F-P, et al. Nisin induces cytotoxicity and apoptosis in human asterocytoma cell line (SW1088). Asian Pac J Cancer Prev. 2018;19(8):2217–22.
Hou Y, Yin Y, Wu G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp Biol Med (Maywood). 2015;240(8):997–1007.
Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17.
Choi BH, Coloff JL. The diverse functions of non-essential amino acids in cancer. Cancers (Basel). 2019;11(5):675.
Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, et al. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. Febs j. 2017;284(11):1726–37.
Lahmy R, Soleimani M, Sanati MH, Behmanesh M, et al. MiRNA-375 promotes beta pancreatic differentiation in human induced pluripotent stem (hiPS) cells. Mol Biol Rep. 2014;41(4):2055–66.
McComish SF, Caldwell MA. Generation of defined neural populations from pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 2018. https://doi.org/10.1098/rstb.2017.0214.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45.
Tukker AM, Wijnolts FMJ, de Groot A, Westerink RHS. Human iPSC-derived neuronal models for in vitro neurotoxicity assessment. Neurotoxicology. 2018;67:215–25.
Yap MS, Nathan KR, Yeo Y, Lim LW, et al. Neural differentiation of human pluripotent stem cells for nontherapeutic applications: toxicology, pharmacology, and in vitro disease modeling. Stem Cells Int. 2015;2015:105172.
Sheridan SD, Surampudi V, Rao RR. Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells Int. 2012;2012:738910.
Zhu L, Gomez-Duran A, Saretzki G, Jin S, et al. The mitochondrial protein CHCHD2 primes the differentiation potential of human induced pluripotent stem cells to neuroectodermal lineages. J Cell Biol. 2016;215(2):187–202.
Lepski G, Jannes C, Nikkhah G, Bischofberger J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front Cell Neurosci. 2013;7:155.
Pan Y, Chen X, Wang S, Yang S, et al. In vitro neuronal differentiation of cultured human embryonic germ cells. Biochem Biophys Res Commun. 2005;327(2):548–56.
Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125–43.
Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61(2):133–68.
Chacon J, Rogers CD. Early expression of Tubulin Beta-III in avian cranial neural crest cells. Gene Expr Patterns. 2019;34:119067.
Hu Q, Agarwal U, Bequette BJ. Gluconeogenesis, non-essential amino acid synthesis and substrate partitioning in chicken embryos during later development. Poult Sci. 2017;96(2):414–24.
Gardner DK. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology. 1998;49(1):83–102.
Shan J, Hamazaki T, Tang TA, Terada N, et al. Activation of the amino acid response modulates lineage specification during differentiation of murine embryonic stem cells. Am J Physiol Endocrinol Metab. 2013;305(3):E325–35.
Bianchi F, Malboubi M, Li Y, George JH, et al. Rapid and efficient differentiation of functional motor neurons from human iPSC for neural injury modelling. Stem Cell Res. 2018;32:126–34.
Corti S, Nizzardo M, Simone C, Falcone M, et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Transl Med. 2012;4(165):165ra2.
Zhang M, Ngo J, Pirozzi F, Sun YP, et al. Highly efficient methods to obtain homogeneous dorsal neural progenitor cells from human and mouse embryonic stem cells and induced pluripotent stem cells. Stem Cell Res Ther. 2018;9(1):67.
Joo NE, Ritchie K, Kamarajan P, Miao D, et al. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305.
Bui VT, Tseng HC, Kozlowska A, Maung PO, et al. Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. 2015;6:576.
Han N, Jia L, Guo L, Su Y, et al. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res Ther. 2020;11(1):61.
Moll GN, Clark J, Chan WC, Bycroft BW, et al. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179(1):135.
Giffard CJ, Ladha S, Mackie AR, Clark DC, et al. Interaction of nisin with planar lipid bilayers monitored by fluorescence recovery after photobleaching. J Membr Biol. 1996;151(3):293–300.
Bartholomae M, Baumann T, Nickling JH, Peterhoff D, et al. Expanding the genetic code of lactococcus lactis and Escherichia coli to incorporate non-canonical amino acids for production of modified lantibiotics. Front Microbiol. 2018;9:657.
Zhou L, van Heel AJ, Montalban-Lopez M, Kuipers OP. Potentiating the activity of nisin against Escherichia coli. Front Cell Dev Biol. 2016;4:7.
Funding
The work was not supported by any Department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The hiPSCs were purchased from The Stem Cell Technology Research Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eftekhari, E., Ghollasi, M., Halabian, R. et al. Nisin and non-essential amino acids: new perspective in differentiation of neural progenitors from human-induced pluripotent stem cells in vitro. Human Cell 34, 1142–1152 (2021). https://doi.org/10.1007/s13577-021-00537-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00537-9