Abstract
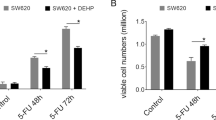
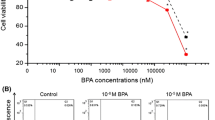
As one of the most prevalent and deadly cancers worldwide, esophageal squamous cell carcinoma (ESCC) can be directly exposed to endocrine-disrupting chemical (EDC). As a potential EDC, diethylhexyl phthalate (DEHP) can trigger the development of various cancers, while the potential effect of DEHP on the ESCC progression was not clear. Our present study revealed that DEHP can trigger the proliferation of ESCC cells and decrease the cisplatin (CDDP) and fluorouracil (5-FU) sensitivity. Mechanistical studies indicated that DEHP can decrease the transcription of PTEN, a well-characterized tumor suppressor, in ESCC cells. Over expression of PTEN can reverse DEHP-regulated ESCC cell proliferation and chemosensitivity. Further, DEHP can increase the expression of HES-1, which can bind with the promoter of PTEN to inhibit its transcription. Collectively, DEHP can increase proliferation while decrease chemosensitivity of ESCC cells via regulation of HES-1/PTEN axis. Further, daily expression of DEHP may be a potent risk factor for ESCC development.







Similar content being viewed by others
Change history
15 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s13577-021-00528-w
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. 2013;61:330–5.
Moon DH, Jeon JH, Yang HC, et al. Intramural metastasis as a risk factor for recurrence in esophageal squamous cell carcinoma. Ann Thorac Surg. 2018;106:249–56.
Lyons TG, Ku GY. Systemic therapy for esophagogastric cancer: targeted therapies. Chin Clin Oncol. 2017;6:48.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16.
Latini G, Del Vecchio A, Massaro M, Verrotti A, De Felice C. Phthalate exposure and male infertility. Toxicology. 2006;226:90–8.
Hopf NB, Berthet A, Vernez D, Langard E, Spring P, Gaudin R. Skin permeation and metabolism of di(2-ethylhexyl) phthalate (DEHP). Toxicol Lett. 2014;224:47–53.
Lopez-Carrillo L, Hernandez-Ramirez RU, Calafat AM, et al. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Persp. 2010;118:539–44.
Zhu MM, Huang C, Ma X, et al. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ Toxicol Phar. 2018;63:29–33.
Chen HP, Lee YK, Huang SY, Shi PC, Hsu PC, Chang CF. Phthalate exposure promotes chemotherapeutic drug resistance in colon cancer cells. Oncotarget. 2018;9:13167–80.
Chou CK, Huang HW, Yang CF, et al. Reduced camptothecin sensitivity of estrogen receptor-positive human breast cancer cells following exposure to di(2-ethylhexyl)phthalate (DEHP) is associated with DNA methylation changes. Environ Toxicol. 2019;34:401–14.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78.
Gopalakrishnan K, Aushev VN, Manservisi F, et al. Gene expression profiles for low-dose exposure to diethyl phthalate in rodents and humans: a translational study with implications for breast carcinogenesis. Sci Rep. 2020;10:7067.
Matejcic M, Iqbal PM. Gene-environment interactions in esophageal cancer. Crit Rev Clin Lab Sci. 2015;52:211–31.
Lin DC, Wang MR, Koeffler HP. Genomic and epigenomic aberrations in esophageal squamous cell carcinoma and implications for patients. Gastroenterology. 2018;154:374–89.
Virolle T, Adamson ED, Baron V, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3:1124–8.
Shen YH, Zhang L, Gan YH, et al. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling—a cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727–36.
Hettinger K, Vikhanskaya F, Poh MK, et al. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14:218–29.
Palomero T, Sulis ML, Cortina M, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–10.
Caldwell JC. DEHP: genotoxicity and potential carcinogenic mechanisms—a review. Mutat Res-Rev Mutat. 2012;751:82–157.
Kim JH. Di(2-ethylhexyl) phthalate promotes lung cancer cell line A549 progression via Wnt/beta-catenin signaling. J Toxicol Sci. 2019;44:237–44.
Crobeddu B, Ferraris E, Kolasa E, Plante I. Di(2-ethylhexyl) phthalate (DEHP) increases proliferation of epithelial breast cancer cells through progesterone receptor dysregulation. Environ Res. 2019;173:165–73.
Vidotto T, Melo CM, Castelli E, Koti M, Dos Reis RB, Squire JA. Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer. 2020;122:1732–43.
Sun ZG, Ji N, Bi MM, Wang S, Liu XY, Wang Z. PTEN gene is infrequently hypermethylated in human esophageal squamous cell carcinoma. Tumor Biol. 2015;36:5849–57.
Jin YY, Chen QJ, Xu K, et al. Involvement of microRNA-141-3p in 5-fluorouracil and oxaliplatin chemo-resistance in esophageal cancer cells via regulation of PTEN. Mol Cell Biochem. 2016;422:161–70.
Peng J, Lv Y, Wu C. Radiation-resistance increased by overexpression of microRNA-21 and inhibition of its target PTEN in esophageal squamous cell carcinoma. J Int Med Res. 2020;48:300060519882543.
Zhang N, Liu JF. MicroRNA (MiR)-301a-3p regulates the proliferation of esophageal squamous cells via targeting PTEN. Bioengineered. 2020;11:972–83.
Wang SC, Lin XL, Wang HY, et al. Hes1 triggers epithelial-mesenchymal transition (EMT)-like cellular marker alterations and promotes invasion and metastasis of nasopharyngeal carcinoma by activating the PTEN/AKT pathway. Oncotarget. 2015;6:36713–30.
Gao F, Huang W, Zhang Y, et al. Hes1 promotes cell proliferation and migration by activating Bmi-1 and PTEN/Akt/GSK3beta pathway in human colon cancer. Oncotarget. 2015;6:38667–80.
Tian T, Fu X, Lu J, et al. MicroRNA-760 inhibits doxorubicin resistance in hepatocellular carcinoma through regulating Notch1/Hes1-PTEN/Akt signaling pathway. J Biochem Mol Toxicol. 2018;32:e22167.
Aghazadeh S, Yazdanparast R. Activation of STAT3/HIF-1alpha/Hes-1 axis promotes trastuzumab resistance in HER2-overexpressing breast cancer cells via down-regulation of PTEN. Biochim Biophys Acta Gen Subj. 2017;1861:1970–80.
Wang F, Liu S, Liu J, et al. SP promotes cell proliferation in esophageal squamous cell carcinoma through the NK1R/Hes1 axis. Biochem Biophys Res Commun. 2019;514:1210–6.
Author information
Authors and Affiliations
Contributions
Conception and design: JZ, BW, JC, WX; acquisition of data: JZ, BW, JC, XZ; analysis and interpretation of data: JZ, XZ, YL, WX; writing, review, and/or revision of the manuscript: JZ, WX.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
No animal or human-related experiment.
Consent for publication
All authors give consent for publishing this study.
Availability of data and material
All data and material are available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, J., Zhang, X., Wen, B. et al. Diethylhexyl phthalate (DEHP) regulates the proliferation and chemosensitivity of esophageal squamous cell carcinoma cells via regulation of PTEN. Human Cell 34, 1153–1162 (2021). https://doi.org/10.1007/s13577-021-00519-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00519-x