Abstract
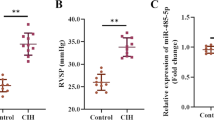
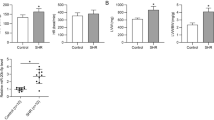
The obstructive sleep apnea syndrome (OSAS) is a common sleep-related breathing disorder and an important cause of refractory hypertension. MicroRNAs (miRNAs) are involved in the development of hypertension, but their role in OSAS with hypertension (OSAS-hypertension) has been little studied. Evidence indicates that miR-126a-3p expression is lower in patients with OSAS-hypertension compared with the patients with OSAS alone. However, its role in the pathogenesis of OSAS-hypertension remains unclear. Therefore, this study aims to investigate the role of miR-126a-3p in OSAS-hypertension and to determine whether HIF-1α is involved in this process. Sprague Dawley rats were exposed to chronic intermittent hypoxia (CIH) for 8 weeks to induce OSAS-hypertension. Rat aortic smooth muscle cells (A7r5) were cultured under hypoxia as an in vitro model. Our results showed that rats exposed to 8 week CIH exhibited decreased miR-126a-3p and increased HIF-1α expression. Furthermore, administration of recombinant adeno-associated virus expressing miR-126a-3p (rAAV-miR-126a) counteracted the CIH-induced systolic blood pressure upregulation, oxidase stress, inflammation, and heart and abdominal aorta vascular remodeling. Moreover, the mechanism was associated with its targeted suppression of HIF-1α. These findings suggest that miR-126a-3p might be a novel potential therapeutic target for the treatment of OSAS-hypertension.




Similar content being viewed by others
References
Fu Q, Wang T, Liang Y, et al. Auditory deficits in patients with mild and moderate obstructive sleep apnea syndrome: a speech syllable evoked auditory brainstem response study. Clin Exp Otorhinolaryngol. 2019;12:58–655.
Powell NB. Contemporary surgery for obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2009;2:107–14.
Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension (Dallas, Tex: 1979). 2011;58:811–7.
Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66:1023–32.
Xiao S-C, He B-T, Steier J, Moxham J, Polkey MI, Luo Y-M. Neural respiratory drive and arousal in patients with obstructive sleep apnea hypopnea. Sleep. 2015;38:941–9.
Nanduri J, Peng Y-J, Yuan G, Kumar GK, Prabhakar NR. Hypoxia-inducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med (Berl). 2015;93:473–80.
Shiina K, Tomiyama H, Takata Y, Chikamori T. Aortic knob width: a possible marker of vascular remodeling in obstructive sleep apnea. J Atheroscler Thromb. 2020;27:499–500.
Shang P, Liu T, Liu W, et al. Telmisartan improves vascular remodeling through ameliorating prooxidant and profibrotic mechanisms in hypertension via the involvement of transforming growth factor-β1. Mol Med Rep. 2017;16:4537–44.
Brown IAM, Diederich L, Good ME, et al. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler Thromb Vasc Biol. 2018;38:1969–85.
Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–29.
Bátkai S, Thum T. MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep. 2012;14:79–877.
Nemecz M, Alexandru N, Tanko G, Georgescu A. Role of microRNA in endothelial dysfunction and hypertension. Curr Hypertens Rep. 2016;18:87.
Santamaria-Martos F, Benítez I, Ortega F, et al. Circulating microRNA profile as a potential biomarker for obstructive sleep apnea diagnosis. Sci Rep. 2019;9:13456–556.
Chistiakov DA, Orekhov AN, Bobryshev YV. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol. 2016;97:47–55.
van Solingen C, Bijkerk R, de Boer HC, Rabelink TJ, van Zonneveld AJ. The role of microRNA-126 in vascular homeostasis. Curr Vasc Pharmacol. 2015;13:341–51.
Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: potential markers of target-organ damage. J Am Soc Hypertens. 2014;8:368–75.
Yang X, Niu X, Xiao Y, Lin K, Chen X. MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: potential diagnostic and early warning markers. Respir Res. 2018;19:194.
Liu Y, Chen C, Qian P, et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat Commun. 2015;6:5988–6088.
Wu CX, Liu Y, Zhang JC. Chronic intermittent hypoxia and hypertension: a review of systemic inflammation and Chinese medicine. Chin J Integr Med. 2013;19:394–400.
Zhang L, Ou X, Zhu T, Lv X. Beneficial effects of estrogens in obstructive sleep apnea hypopnea syndrome. Sleep Breath Schlaf Atmung. 2020;24:7–13.
Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94.
Cruz de Carvalho MH. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav. 2008;3:156–65.
Ferreira I, Hovind P, Schalkwijk CG, Parving HH, Stehouwer CDA, Rossing P. Biomarkers of inflammation and endothelial dysfunction as predictors of pulse pressure and incident hypertension in type 1 diabetes: a 20 year life-course study in an inception cohort. Diabetologia. 2018;61:231–41.
van Dooren FE, Schram MT, Schalkwijk CG, et al. Associations of low grade inflammation and endothelial dysfunction with depression—the Maastricht study. Brain Behav Immun. 2016;56:390–6.
Sun H-J, Ren X-S, Xiong X-Q, et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8:e3074–e31743174.
Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293:L417–L428428.
Shang P, Liu W, Liu T, et al. Acetyl-11-keto-β-boswellic acid attenuates prooxidant and profibrotic mechanisms involving transforming growth factor-β1, and improves vascular remodeling in spontaneously hypertensive rats. Sci Rep. 2016;6:39809.
Li H, Zhang X, Wang F, et al. MicroRNA-21 lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation. Circulation. 2016;134:734–51.
Chu M, Qin S, Wu R, et al. Role of MiR-126a-3p in endothelial injury in endotoxic mice. Crit Care Med. 2016;44:e639–e650650.
Olivieri F, Spazzafumo L, Bonafè M, et al. MiR-21-5p and miR-126a-3p levels in plasma and circulating angiogenic cells: relationship with type 2 diabetes complications. Oncotarget. 2015;6:35372–82.
Daiber A, Xia N, Steven S, et al. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci. 2019;20:187.
Zhang B-F, Jiang H, Chen J, Guo X, Hu Q, Yang S. KDM3A inhibition attenuates high concentration insulin-induced vascular smooth muscle cell injury by suppressing MAPK/NF-κB pathways. Int J Mol Med. 2018;41:1265–74.
Acknowledgements
We are grateful to the Department of Respiratory Medicine and all the investigators for their support and hard work during the study.
Funding
This work was supported by the Science and Technology Department of Jiangxi Province (20161BBG70231).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, L., Liao, X., Zhu, G. et al. miR-126a-3p targets HIF-1α and alleviates obstructive sleep apnea syndrome with hypertension. Human Cell 33, 1036–1045 (2020). https://doi.org/10.1007/s13577-020-00404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00404-z