Abstract
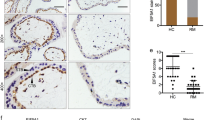
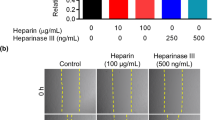
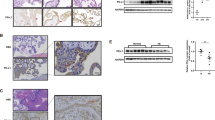
Hepatocyte growth factor activator inhibitor type 1/serine protease inhibitor Kunitz type 1 (HAI-1/SPINT1) is a membrane-bound Kunitz-type serine protease inhibitor that is abundantly expressed on the surface of cytotrophoblasts, and is critically required for the formation of the placenta labyrinth in mice. HAI-1/SPINT1 regulates several membrane-associated cell surface serine proteases, with matriptase being the most cognate target. Matriptase degrades extracellular matrix protein such as laminin and activates other cell surface proteases including prostasin. This study aimed to analyze the role of HAI-1/SPINT1 in pericellular proteolysis of trophoblasts. In HAI-1/SPINT1-deficient mouse placenta, laminin immunoreactivity around trophoblasts was irregular and occasionally showed an intense punctate pattern, which differed significantly from the linear distribution along the basement membrane observed in wild-type placenta. To explore the molecular mechanism underlying this observation, we analyzed the effect of HAI-1/SPINT1 knock down (KD) on pericellular proteolysis in the human trophoblast cell line, BeWo. HAI-1/SPINT1-KD BeWo cells had increased amounts of cellular laminin protein and decreased laminin degradation activity in the culture supernatant. Subsequent analysis indicated that cell-associated matriptase was significantly decreased in KD cells whereas its mRNA level was not altered, suggesting an enhanced release and/or dislocation of matriptase in the absence of HAI-1/SPINT1. Moreover, prostasin activation and pericellular total serine protease activities were significantly suppressed by HAI-1/SPINT1 KD. These observations suggest that HAI-1/SPINT1 is critically required for the cell surface localization of matriptase in trophoblasts, and, in the absence of HAI-1/SPINT1, physiological activation of prostasin and other protease(s) initiated by cell surface matriptase may be impaired.






Similar content being viewed by others
References
Shimomura T, Denda K, Kitamura A, et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:6370–6.
Eigenbrot C, Ganesan R, Kirchhofer D. Hepatocyte growth factor activator (HGFA): molecular structure and interactions with HGFA inhibtor-1 (HAI-1). FEBS J. 2010;277:2215–22.
Kataoka H, Kawaguchi M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. FEBS J. 2010;277:2230–7.
Kawaguchi T, Qui L, Shimomura T, et al. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:27558–64.
Fukushima T, Kawaguchi M, Yamasaki M, Tanaka H, Yorita K, Kataoka H. Hepatocyte growth factor activator inhibitor type 1 suppresses metastatic pulmonary colonization of pancreatic carcinoma cells. Cancer Sci. 2011;102:407–13.
Kataoka H, Suganuma T, Shimomura T, et al. Distribution of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in human tissues. Cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J Histochem Cytochem. 1999;47:673–82.
Kataoka H, Meng JY, Itoh H, et al. Localization of hepatocyte growth factor activator inhibitor type 1 in Langhans’ cells of human placenta. Histochem Cell Biol. 2000;114:469–75.
Tanaka H, Fukushima T, Yorita K, Kawaguchi M, Kataoka H. Tissue injury alters the site of expression of hepatocyte growth factor activator inhibitor type 1 in bronchial epithelial cells. Hum Cell. 2009;22:11–7.
Tanaka H, Nagaike K, Takeda N, et al. Hepatocyte growth factor activator inhibitor type 1 (HAI-1) is required for branching morphogenesis in the chorioallantoic placenta. Mol Cell Biol. 2005;25:5687–98.
Nagaike K, Kawaguchi M, Takeda N, et al. Defect of hepatocyte growth factor activator inhibitor type 1/serine protease inhibitor, Kunitz type 1 (Hai-1/Spint1) leads to ichthyosis-like condition and abnormal hair development in mice. Am J Pathol. 2008;173:1464–75.
Kawaguchi M, Takeda N, Hoshiko S, et al. Membrane-bound serine protease inhibitor HAI-1 is required for maintenance of intestinal epithelial integrity. Am J Pathol. 2011;179:1815–26.
Delaria KA, Muller DK, Marlor CW, et al. Characterization of placental bikunin, a novel human serine protease inhibitor. J Biol Chem. 1997;272:12209–14.
Fan B, Brennan J, Grant D, Peale F, Rangell L, Kirchhofer D. Hepatocyte growth factor activator inhibitor-1 (HAI-1) is essential for the integrity of basement membranes in the developing placental labyrinth. Dev Biol. 2007;303:222–30.
Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–56.
Antalis TM, Buzza MS, Hodge KM, Hopper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–46.
Chen YW, Eckert RD, Johnson MD, Lin CY. The transmembrane serine protease matriptase: implications for cellular biology and human diseases. J Med Sci. 2012;32:97–108.
Satomi S, Yamasaki Y, Tsuzuki S, Hitomi Y, Iwanaga T, Fushiki T. A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem Biophys Res Commun. 2001;287:995–1002.
Hurst NJ Jr, Najy AJ, Ustach CV, Movilla L, Kim HR. Platelet-derived growth factor-C (PDGF-C) activation by serine proteases: implication for breast cancer progression. Biochem J. 2012;441:909–18.
Ustach CV, Huamng W, Conley-LaComb MK, et al. A novel signaling axis of matriptase/PDGF-D/b-PDGFR in human prostate cancer. Cancer Res. 2010;70:9631–40.
List K, Currie B, Scharschmidt TC, et al. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007;282:36714–23.
Miller GS, List K. The matriptase-prostasin proteolytic cascade in epithelial development and pathology. Cell Tissue Res. 2012;. doi:10.1007/s00441-012-1348-1.
Donaldson SH, Hirsh A, Li DC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–45.
Chen YW, Wang JK, Chou FP, et al. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 during epidermal differentiation. J Biol Chem. 2010;285:31755–62.
Xu H, Xu Z, Tseng IC, et al. Mechanisms for the control of matriptase activity in the absence of sufficient HAI-1. Am J Physiol Cell Physiol. 2012;302:C453–62.
Oberst MD, Chen LY, Kiyomiya K, et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005;289:C462–70.
Lee MS, Tseng IC, Wang Y, et al. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293:C95–105.
Kirchhofer D, Peek M, Lipari MT, Billeci K, Fan B, Moran P. Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and HAI-2. FEBS Lett. 2005;579:1945–50.
Hashimoto T, Kato M, Shimomura T, Kitamura N. TMPRSS13, a type II transmembrane serine protease, is inhibited by hepatocyte growth factor activator inhibitor type 1 and activates pro-hepatocyte growth factor. FEBS J. 2010;277:4888–900.
Kato M, Hashimoto T, Shimomura T, Kataoka H, Ohi H, Kitamura N. Hepatocyte growth factor activator inhibitor type 1 inhibits protease activity and proteolytic activation of human airway trypsin-like protease. J Biochem. 2012;151:179–87.
Fan B, Wu TD, Li W, Kirchhofer D. Identification of hepatocyte growth factor activator inhibitor-1B as a potential physiological inhibitor of prostasin. J Biol Chem. 2005;280:34513–20.
Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28:1231–6.
del Mónaco S, Assef Y, Kotsias BA. Epithelial sodium channel in a human trophoblast cell line (BeWo). J Membr Biol. 2008;223:127–39.
Tseng IC, Xu H, Chou FP, et al. Matriptase activation, an early cellular response to acidosis. J Biol Chem. 2010;285:3261–70.
Fukushima T, Tezuka T, Shimomura T, Nakano S, Kataoka H. Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem. 2007;282:18634–44.
Baba T, Kawaguchi M, Fukushima T, et al. Loss of membrane-bound serine protease inhibitor HAI-1 induces oral squamous cell carcinoma cells’ invasiveness. J Pathol. 2012;228:181–92.
Szabo R. Uzzun Sales K, Kosa P, et al. Reduced prostasin (CAP1/PRSS8) activity eliminates HAI-1 and HAI-2 deficiency-associated developmental defects by preventing matriptase activation. PLoS Genet. 2012;8:e1002937.
Friis S, Godiksen S, Bornholdt J, et al. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem. 2011;286:5793–802.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research no. 24390099 and no. 23790250 from the Ministry of Education, Science, Sports and Culture, Japan.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kohama, K., Kawaguchi, M., Fukushima, T. et al. Regulation of pericellular proteolysis by hepatocyte growth factor activator inhibitor type 1 (HAI-1) in trophoblast cells. Human Cell 25, 100–110 (2012). https://doi.org/10.1007/s13577-012-0055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-012-0055-2