Abstract
Introduction
Health-related quality of life (HRQoL) is an important part of the clinical assessment and management of psoriasis. Few studies have investigated the effects of withdrawing treatment on the relationship between HRQoL and objective clinical manifestations of psoriasis. This post hoc subanalysis of a clinical trial (REVEAL) examined the relationship of HRQoL [assessed with the Dermatology Life Quality Index (DLQI)] and objective disease activity [assessed with the Psoriasis Area and Severity Index (PASI)] among patients before and after they underwent protocol-mandated discontinuation of psoriasis therapy.
Methods
Adult patients with moderate-to-severe plaque psoriasis who received adalimumab from baseline and had 75% or greater improvement in the PASI score at weeks 16 and 33 were re-randomized to adalimumab 40 mg or placebo every other week from weeks 33 to 52. DLQI and PASI scores were compared at baseline (week 0), early in treatment (week 4), directly before randomized withdrawal (week 33), and up to 19 weeks after treatment discontinuation (week 52; last observations carried forward). Correlations between DLQI total score and PASI score at week 4 and week 52 were modeled by linear regression.
Results
In the patients (N = 240) who underwent protocol-mandated discontinuation of psoriasis treatment after achieving PASI 75 response, mean PASI scores at week 52 were lower (i.e., better) compared with week 4, yet mean DLQI scores were higher (i.e., worse). An approximately twofold disproportionately greater degree of worsening of DLQI score compared with the degree of worsening of PASI was observed while patients underwent discontinuation of therapy (week 52) compared with early in treatment (week 4). There was a significant interaction (P < 0.0001) between the PASI–DLQI correlation and study period (week 4 or 52).
Conclusion
Discontinuing therapy in patients who initially responded to treatment, as seen in this analysis with adalimumab, disproportionately worsened patient-reported HRQoL relative to the worsening of PASI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a major inflammatory disease with significant associated comorbidities, including cardiovascular disease, inflammatory bowel disease, metabolic syndrome, and psoriatic arthritis [1]. Health-related quality of life (HRQoL) is increasingly recognized as an important element of the overall clinical assessment of psoriasis rather than simply an adjunct to objective measures of disease [2]. The Dermatology Life Quality Index (DLQI) is a short, self-administered, dermatology-specific questionnaire that is the most commonly used tool to evaluate HRQoL in psoriasis clinical research [2, 3]. Objective clinical manifestations of psoriasis are commonly assessed with the Psoriasis Area and Severity Index (PASI), a validated scale that has been demonstrated to quantify clinical improvement in response to treatment [4, 5].
Although it is difficult to correlate absolute values of the PASI and DLQI, several studies have explored the relationship between reductions in the objective severity of psoriasis as measured with the PASI and improvements in HRQoL as measured with the DLQI [6]. In secondary analyses of 1,469 patients with moderate-to-severe plaque psoriasis from two clinical trials of the tumor necrosis factor (TNF) inhibitor adalimumab, a 90% or greater improvement in the PASI (PASI 90 response) was associated with a greater improvement in the DLQI than that associated with a 75% or greater improvement in the PASI (PASI 75 response) [7]. Further, a study of the TNF inhibitor infliximab for severe plaque psoriasis demonstrated that the percent change in the PASI and the percent change in the DLQI for the combined infliximab and placebo groups were strongly correlated (Spearman’s correlation of 0.61) [8]. Additionally, patients who achieved a PASI 75 response had a greater mean improvement in the DLQI (81%) compared with patients who achieved 50–75% improvement in the PASI (60% improvement in the DLQI). Katugampola et al. [9] examined associations between the PASI and the DLQI in multiple clinical trials of four biologics (etanercept, infliximab, efalizumab, and alefacept) and reported that improvement in the DLQI paralleled improvement in the PASI 75 with treatment. Conversely, patients who had responded to ustekinumab treatment (PASI 75 responders) and were re-randomized to placebo during a withdrawal period exhibited rapid worsening of DLQI scores in parallel with loss of the PASI response; however, the association between DLQI and PASI scores was not formally analyzed [10]. To date, no studies have compared the correlation between PASI and DLQI scores during effective disease control and after withdrawal of therapy.
In a double-blind, randomized, controlled phase 3 trial (Randomized Controlled EValuation of Adalimumab Every Other Week Dosing in Moderate to Severe Psoriasis TriAL [REVEAL]; Clinicaltrials.gov #NCT00237887), patients who underwent a protocol-mandated discontinuation of treatment at week 33 experienced a loss of adequate response [defined as a <50% reduction in PASI score relative to baseline (week 0) and a ≥6-point increase in the PASI score compared with week 33] at a significantly higher rate than patients who were randomized to continue active treatment (28% vs. 5%; P < 0.001) [11]. We hypothesized that patients who had achieved a clinical response in REVEAL and whose treatment was then discontinued would experience a worsening of dermatology-specific HRQoL out of proportion to the worsening of the objective signs of disease.
Methods
Study Design
This was a post hoc analysis of a phase 3, multicenter, 52-week, double-blind, placebo-controlled trial (REVEAL) performed at 81 sites across the USA and Canada (Clinicaltrials.gov #NCT00237887) [11]. The protocol was approved by the institutional review board of each participating medical center. The REVEAL study consisted of three treatment periods (Fig. 1). In period A (weeks 0–16), patients were randomized 2:1 using an interactive voice response system to receive double-blind subcutaneous injections of adalimumab or placebo. An initial dose of adalimumab (80 mg) or placebo was administered at week 0, followed by 40 mg adalimumab or placebo every other week (eow) from weeks 1 to 15. Patients who achieved PASI 75 at week 16 were eligible to enter the open-label period (period B, weeks 16–33). To maintain blinding at week 16, patients who had received placebo during period A received two injections of adalimumab (40 mg), while patients who had received adalimumab were given two injections of placebo. All patients received adalimumab (40 mg eow) from weeks 17 to 31. Patients who achieved PASI 75 responses at week 33 were eligible to enter period C (weeks 33–52). Patients originally randomized to adalimumab in period A were then re-randomized 1:1 to receive double-blind adalimumab or placebo from weeks 33 to 52 (period C). Patients who received placebo in period C are the focus of this analysis.
REVEAL study design. Patients originally randomized to adalimumab in period A (weeks 0–16) who achieved PASI 75 responses at week 16 (after double-blind treatment) and week 33 [after open-label period B (weeks 16–33)] were then re-randomized to double-blind adalimumab or placebo in period C (weeks 33–52). This analysis examined the cohort of 240 patients treated with placebo in period C. *40 mg eow from week 1 after 80 mg at week 0; †40 mg eow from week 17 after 80 mg at week 16. Adapted with permission from Menter et al. [11]. DBPC double-bind, placebo controlled, eow every other week, OLE open-label extension, PASI Psoriasis Area and Severity Index, REVEAL Randomized Controlled EValuation of Adalimumab Every Other Week Dosing in Moderate to Severe Psoriasis TriAL
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Patients
Males and females aged 18 years or older with a clinical diagnosis of moderate-to-severe plaque psoriasis (affected body surface area ≥10%) for at least 6 months were eligible for the REVEAL study [11]. PASI scores of 12 or greater and Physician’s Global Assessment of at least “moderate” were required for eligibility. Key exclusion criteria included previous exposure to any systemic anti-TNF therapy and the inability to discontinue phototherapy or topical or systemic therapies. All patients provided written informed consent.
Efficacy Assessments
The DLQI consists of four components: symptoms and feelings (DLQIS); daily activities (DLQID); leisure (DLQIL); and personal relationships (DLQIP). DLQI total scores may range from 0 to 30, with a higher score indicating poorer HRQoL. A DLQI total score of more than 5 indicates a moderate effect of psoriasis on patient HRQoL; a DLQI score of more than 10 indicates a very large effect; and a DLQI score of more than 20 indicates an extremely large effect [12]. Thus, a DLQI total score of more than 10 supports the need for a more aggressive intervention. PASI scores may range from 0 to 72, with higher scores indicating greater disease severity. Post hoc comparisons of DLQI and PASI scores were evaluated at baseline, early in treatment (week 4), at the last point when all patients had at least a PASI 75 response (week 33), and after randomized treatment discontinuation (up to 19 weeks after discontinuation).
Statistical Analysis
Patients who received adalimumab during period A, achieved PASI 75 responses at week 16 and week 33, and were subsequently re-randomized to placebo during period C were the focus of this subanalysis. Missing data were imputed using the last observation carried forward. The correlation between DLQI component or total score and PASI score at weeks 4 and 52 was analyzed by linear regression using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). No other statistical hypothesis testing was conducted for this post hoc analysis.
Results
Patients
Demographic and baseline disease activity characteristics of patients (N = 240) in the subanalysis population (Table 1) appeared generally similar to those of patients in the overall study population [11]. The mean age of patients was approximately 43 years; most patients were male and nearly all were white. Baseline disease characteristics were consistent with moderate-to-severe psoriasis; the mean DLQI score at baseline (11.6) was >10, suggesting that, on average, psoriasis had a very large effect on HRQoL.
PASI Response
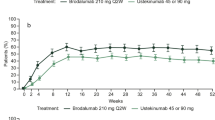
The proportion of patients with PASI 75, PASI 90, or PASI 100 responses decreased after discontinuation of therapy (Fig. 2). All patients had PASI 75 responses at week 33, but most of these patients lost PASI 75 response at week 52, following discontinuation of treatment. Similarly, the proportion of patients achieving a PASI 90 response decreased from 71.7% at week 33 to 20.3% at week 52, and the PASI 100 response rate decreased from 37.9% at week 33 to 8.4% at week 52. Thus, discontinuation of therapy at week 33 resulted in recurrence of psoriasis symptoms by week 52.
Proportion of patients achieving PASI 75, PASI 90, and PASI 100 responses over time in the ITT population (LOCF). Patients who achieved PASI 75 responses with adalimumab at week 33 and then discontinued treatment (received placebo) until week 52 are presented. For week 52, only patients who had at least 1 post-week 33 assessment were included in the LOCF analysis. ITT intent to treat, LOCF last observation carried forward, PASI Psoriasis Area and Severity Index
PASI vs. DLQI
Mean PASI and DLQI scores at baseline, week 4, week 33, and week 52 are reported in Fig. 3. Mean PASI scores decreased from baseline to week 4 and continued to decline until week 33, indicating improvement in psoriasis symptoms. Mean PASI score increased from week 33 to week 52, indicating recurrence of disease symptoms upon discontinuation of treatment. Mean DLQI scores followed a similar pattern. However, although the mean PASI score at week 52 (6.7) remained lower than the PASI score at week 4 (8.0), the mean DLQI score at week 52 (5.3) was higher than at week 4 (4.3). Therefore, at the end of the study, PASI scores remained lower (indicating an improvement) compared with early treatment, whereas the mean DLQI score was worse at the end of the study compared with a time point early in treatment, suggesting a disproportionate worsening of HRQoL relative to the severity of psoriasis.
To examine the association between the PASI and DLQI, the correlation between PASI scores and DLQI scores was evaluated by linear regression early in treatment (week 4) and 19 weeks after discontinuation of therapy (week 52). For every unit increase in PASI score, there was a disproportionately larger increase (i.e., worsening) in DLQI total score at week 52 compared with week 4 (Fig. 4). The slope of the regression line for DLQI versus PASI was 0.5094 at week 52 and 0.1978 at week 4, indicating that each unit change in PASI was associated with approximately twice as much change in DLQI at week 52 compared with the change at week 4. Linear regression modeling showed a significant interaction (P < 0.0001) between study period (week 4 or week 52) and the relationship of PASI to DLQI, indicating that the correlation between PASI and DLQI was different early in treatment (week 4) from the correlation 19 weeks after discontinuation of treatment (week 52). The disproportionate worsening in DLQI scores was less apparent for patients with PASI 75 responses. Patients who experienced substantial worsening of disease (e.g., <PASI 50) had the largest difference in DLQI scores between week 4 and week 52 (Fig. 5). Changes in these patients made the largest contribution to the mean worsening of DLQI scores. Correlations of individual DLQI component scores with PASI also differed before and after discontinuation of therapy. The slope of the regression line for DLQID versus PASI was 0.0413 at week 4 and 0.1153 at week 52; DLQIL versus PASI was 0.0384 at week 4 and 0.0849 at week 52; DLQIP versus PASI was 0.0281 at week 4 and 0.0578 at week 52; and DLQIS versus PASI was 0.0644 at week 4 and 0.1704 at week 52. Thus, the correlations of DLQIS and DLQID with PASI exhibited the most striking differences between a time point early in treatment and after discontinuation.
Discussion
Within 19 weeks after therapy was discontinued per protocol in patients who had responded well to treatment, PASI and DLQI scores showed recurrence of psoriasis symptoms and increased impairment of HRQoL. A post hoc analysis demonstrated that DLQI total scores worsened disproportionately relative to PASI scores, a difference that reached statistical significance; thus, HRQoL worsened disproportionately relative to the objective signs of disease recurrence. The patterns of correlations of DLQI component scores with PASI scores before and after treatment discontinuation supported the results obtained for the DLQI total score. Although the reason for the asymmetry between PASI and DLQI cannot be determined from the results of this analysis, we hypothesize that it occurred because patients were distressed by the recurrence of psoriasis symptoms that had been effectively controlled. Even mild disease recurrence may have had a negative effect on DLQI scores, perhaps because patients recalled the previous deleterious effects of more severe disease activity on their HRQoL. The psychological impact of psoriasis on patients is well documented. Patients with psoriasis have higher levels of anxiety and depression than healthy controls and often experience emotions of shame, worry, anger, and annoyance [13–15]. Recently, Ghajarzadeh et al. [16] reported significant correlations between DLQI scores and Beck Depression Inventory (BDI) scores in patients with psoriasis. Patients with psoriasis had BDI scores indicative of mild-to-moderate depression and DLQI scores demonstrating impaired HRQoL.
The results of this exploratory analysis of randomized withdrawal of adalimumab are consistent with a substantial link between treatment response and patient HRQoL; we hypothesize that findings would be similar with discontinuation of other treatments. The results of the current study highlight the importance of continuous therapy in patients with psoriasis. Even temporary discontinuations of treatment may be inadvisable in patients who have achieved disease control. Conversely, in the authors’ clinical experience, the erosion of HRQoL after treatment interruption may be a primary factor in influencing patients to reinitiate therapy. In support of these considerations, a recent systematic review concluded that continuous therapy is preferred for maintenance of disease control and HRQoL [17].
This study benefited from a double-blind design that may have minimized bias by masking assignment to continue adalimumab or receive placebo; this was probably particularly important for a subjective, patient-reported outcome such as the DLQI. Additional strengths were that the study was conducted at a large number of centers and that many patients were analyzed. Limitations of the study are that it was a post hoc analysis and, therefore, potentially subject to bias. Additionally, the comparison of PASI and DLQI scores was not evaluated beyond the relatively short period of 19 weeks after discontinuation of treatment. Finally, inherent limitations of the DLQI and PASI have been reported. Problems with the DLQI include inadequate measurement of mild illness, interpretability that has been rated only “acceptable,” and differential item functioning by disease, age, and sex [3, 18]. Although the PASI is the most thoroughly validated scale for psoriasis and is used extensively in clinical trials [4], it has been criticized for having a nonlinear scale, poor sensitivity, and lack of standardization for translation into corresponding clinical severity categories [19]. PASI scores on the lower end of the range (PASI <10) are less meaningful, so comparisons with DLQI scores among patients with low PASI scores may be more difficult. Furthermore, consistency and reliability of the PASI are influenced by the experience level of the physician [20]. Due to these limitations, this analysis must be considered exploratory and hypothesis generating; a prospective study would be needed to confirm the findings.
Conclusion
Discontinuing therapy, as observed in this analysis with adalimumab, has several negative effects in patients with psoriasis, including a loss of objective response and a disproportionately large negative impact on HRQoL. Interruption of treatment will likely result in an increase of psoriasis symptoms and a disproportionate decrease in HRQoL.
References
Mrowietz U, Elder JT, Barker J. The importance of disease associations and concomitant therapy for the long-term management of psoriasis patients. Arch Dermatol Res. 2006;298:309–19.
Basra MK, Hussain S. Application of the Dermatology Life Quality Index in clinical trials of biologics for psoriasis. Chin J Integr Med. 2012;18:179–85.
Bronsard V, Paul C, Prey S, et al. What are the best outcome measures for assessing quality of life in plaque type psoriasis? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):17–22.
Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):10–6.
Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66:369–75.
Reich K, Griffiths CE. The relationship between quality of life and skin clearance in moderate-to-severe psoriasis: lessons learnt from clinical trials with infliximab. Arch Dermatol Res. 2008;300:537–44.
Revicki DA, Willian MK, Menter A, Saurat JH, Harnam N, Kaul M. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology. 2008;216:260–70.
Feldman SR, Gordon KB, Bala M, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. Br J Dermatol. 2005;152:954–60.
Katugampola RP, Lewis VJ, Finlay AY. The Dermatology Life Quality Index: assessing the efficacy of biological therapies for psoriasis. Br J Dermatol. 2007;156:945–50.
Kimball AB, Gordon KB, Fakharzadeh S, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012;166:861–72.
Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–15.
Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol. 2005;125:659–64.
Golpour M, Hosseini SH, Khademloo M, et al. Depression and anxiety disorders among patients with psoriasis: a hospital-based case–control study. Dermatol Res Pract. 2012;2012:381905.
Palijan TZ, Kovacevic D, Koic E, Ruzic K, Dervinja F. The impact of psoriasis on the quality of life and psychological characteristics of persons suffering from psoriasis. Coll Antropol. 2011;35(Suppl 2):81–5.
Sampogna F, Tabolli S, Abeni D. Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm Venereol. 2012;92:299–303.
Ghajarzadeh M, Ghiasi M, Kheirkhah S. Associations between skin diseases and quality of life: a comparison of psoriasis, vitiligo, and alopecia areata. Acta Med Iran. 2012;50:511–5.
Brezinski EA, Armstrong AW. Off-label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS ONE. 2012;7:e33486.
Twiss J, Meads DM, Preston EP, Crawford SR, McKenna SP. Can we rely on the Dermatology Life Quality Index as a measure of the impact of psoriasis or atopic dermatitis? J Invest Dermatol. 2012;132:76–84.
Berth-Jones J, Grotzinger K, Rainville C, et al. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment. Br J Dermatol. 2006;155:707–13.
Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563–9.
Acknowledgments
AbbVie Inc. funded this study and participated in the study design, data collection, data management, data analysis, and preparation of the manuscript. All authors had full access to the data and analyses; were involved in the writing, review of, and decision to submit the manuscript for publication; and vouch for the accuracy and completeness of the data analyses and results presented. The data within this manuscript were previously presented at the 70th Annual Meeting of the American Academy of Dermatology in San Diego, CA, USA, March 16–20, 2012. Medical writing support was provided by Amanda Sheldon, PhD, of Complete Publication Solutions, LLC; this support was funded by AbbVie Inc.
Conflict of interest
Yves Poulin has participated as an investigator, speaker, and as a member of advisory boards for and has received grant funding and honoraria from AbbVie Inc., Amgen, and Centocor/Janssen-Ortho/Johnson & Johnson. Yves Poulin has also received grant funding as an investigator for Celgene, Eli Lilly, LEO Pharma, Merck, Novartis, and Pfizer. Pranav Sheth has served as a speaker for AbbVie and LEO Pharma, and has served as principal investigator on clinical trials supported by AbbVie; funding for these studies went directly to his institution. Pranav Sheth has also participated as a member on advisory boards for AbbVie and Amgen. Yihua Gu and Henrique D. Teixeira are employees of AbbVie and may hold AbbVie stock or stock options.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: Clinicaltrials.gov #NCT00237887.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Poulin, Y., Sheth, P., Gu, Y. et al. Health-Related Quality of Life Worsens Disproportionately to Objective Signs of Psoriasis After Withdrawal of Adalimumab Therapy. Dermatol Ther (Heidelb) 4, 33–42 (2014). https://doi.org/10.1007/s13555-014-0043-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-014-0043-4