Abstract
Background
Cancer cachexia is the progressive loss of skeletal muscle protein that contributes significantly to cancer morbidity and mortality. Evidence of antioxidant attenuation and the presence of oxidised proteins in patients with cancer cachexia indicate a role for oxidative stress. The level of oxidative stress in tissues is determined by an imbalance between reactive oxygen species production and antioxidant activity. This study aimed to investigate the superoxide generating NADPH oxidase (NOX) enzyme and antioxidant enzyme systems in murine adenocarcinoma tumour-bearing cachectic mice.
Methods
Superoxide levels, mRNA levels of NOX enzyme subunits and the antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidise (GPx) and catalase was measured in the skeletal muscle of mice with cancer and cancer cachexia. Protein expression levels of NOX enzyme subunits and antioxidant enzyme activity was also measured in the same muscle samples.
Results
Superoxide levels increased 1.4-fold in the muscle of mice with cancer cachexia, and this was associated with a decrease in mRNA of NOX enzyme subunits, NOX2, p40phox and p67phox along with the antioxidant enzymes SOD1, SOD2 and GPx. Cancer cachexia was also associated with a 1.3-fold decrease in SOD1 and 2.0-fold decrease in GPx enzyme activity.
Conclusion
Despite increased superoxide levels in cachectic skeletal muscle, NOX enzyme subunits, NOX2, p40phox and p67phox, were downregulated along with the expression and activity of the antioxidant enzymes. Therefore, the increased superoxide levels in cachectic skeletal muscle may be attributed to the reduction in the activity of endogenous antioxidant enzymes.
Similar content being viewed by others
1 Introduction
Cancer cachexia is a condition of progressive muscle wasting that develops as a secondary condition in response to tumour growth [1]. While cachexia develops in a wide range of pathologies including anorexia nervosa, acquired immunodeficiency syndrome, amyotrophic lateral sclerosis, congestive heart failure and various malignant cancers, cancer cachexia has been reported to develop at a faster rate than any other cachectic condition [1]. Approximately half of all patients with cancer experience cachexia and almost a third of mortalities are estimated to result from cachexia [2]. Tumour growth induces a specific catabolic response in skeletal muscle that causes the accelerated loss of protein, characterised by the significant loss of body weight [3]. Animal tumour models have been used to investigate cancer cachexia, developing up to 30% loss in body weight after tumour cell implantation. NMRI mice implanted with murine adenocarcinoma (MAC) 16 present with solid tumour growth and cachexia [4], as have been shown in rats bearing the Yoshida AH-130 hepatoma [5], characteristic of the human cancer cachectic condition.
An abundance of evidence exists for the induction of the ubiquitin (Ub)-proteasome proteolytic pathway in the development of cancer cachexia; however, evidence of protein oxidation in cancer cachectic patients suggests the involvement of reactive oxygen species (ROS) [6]. Studies in both humans and animals with cancer cachexia have shown the presence of oxidised proteins [6]. Furthermore, cancer patients have been shown to present with elevated levels of serum ROS and lower antioxidant levels [7], indicative of a pro-oxidative shift. Similarly, increased levels of ROS have been shown in cachectic tumour-bearing rats, with no compensatory response from antioxidant enzymes [5]. Although the catabolic mechanism leading to the severe muscle wasting associated with cancer development remains relatively unknown, there is evidence to suggest a role for ROS [7].
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is a multicomponent enzyme that when activated catalyzes the production of superoxide anion (O −2 ). The NOX2 enzyme consists of five subunits segregated into membrane bound, NOX2 (gp91phox) and p22phox, and cytosolic, p67phox, p47phox and p40phox, units. On stimulation, the protein components of the NOX2 enzyme assemble, forming the active oxidase and the production of O −2 [8]. It has been apparent in recent years that non-phagocytes possess homologues to NOX2, and that these NOX enzymes may activate in a similar manner to the well-established phagocyte NOX2 enzyme [9]. Currently, there are five known NOX homologues (NOX1-5) found to function in a variety of tissues, with the expression of both NOX2 and NOX4 enzyme subunits in skeletal muscle tissue, although its function in this particular cell system remains undefined [10]. Despite the unknown functionality of the NOX enzymes in skeletal muscle tissue, O −2 generated from NOX has been implicated in progressive skeletal muscle damage [11]. The abundance of evidence for NOX enzyme-generated O −2 in disease [11], establishes its potential role in the catabolic skeletal muscle wasting in cancer cachexia.
It is well-known that ROS are generated in skeletal muscle as an essential by-product of cellular metabolism and oxidative enzymes [12]. Due to the high metabolic activity and oxidative capacity of skeletal muscle, the antioxidant system is a crucial component for the maintenance of cellular oxidative homeostasis [13]. With the knowledge that ROS are likely catabolic factors in the development of cancer cachexia, antioxidant enzymes are of equal importance for their crucial role in the maintenance of cellular oxidative homeostasis and the protection against harmful ROS [13]. O −2 is dismutated by the O −2 specific antioxidant enzyme, superoxide dismutase (SOD). Three distinct forms of SOD exist in the intracellular cytoplasmic compartment (SOD1), mitochondria (SOD2) and extracellular matrix (SOD3) [14]. The SOD enzymes catalyse the conversion of O −2 to H2O2, which is further converted by catalase and glutathione peroxidise (GPx) scavenging [14]. A depression in these antioxidant enzyme systems can consequently lead to an imbalance in cellular oxidation and oxidative tissue damage [13], which has been shown particularly in response to disease [5]. Endogenous antioxidants such as SOD and GPx are low in cancer patients presenting with high levels of ROS, indicating the importance of tight regulation from endogenous antioxidants [7].
The Ub-proteasome proteolytic pathway has been established in cancer-induced cachexia. In addition, tumour-derived factors such as TNF-α and proteolysis-inducing factor (PIF) are well recognised in the cancer cachectic condition [15]. What is yet to be elucidated, however, are the downstream mediators of this complex system, in response to tumour growth and development of cancer cachexia. A study by Russell et al. [16] proposed a cachectic pathway of increased Ub gene expression, downstream of NOX-generated ROS production, establishing a potential role for the NOX enzymes in mediating skeletal muscle atrophy through the Ub-proteasome proteolytic pathway.
Therefore, this study aims to investigate the O −2 generating NOX and antioxidant enzyme systems in a well-established animal model of cancer cachexia. In particular, this study aims to determine O −2 levels and NOX enzyme system expression (NOX2, NOX4, p22phox, p40phox, p47phox, p67phox and Rac1) in the skeletal muscle of cachectic (MAC16) tumour-bearing mice compared to non-cachectic (MAC13) tumour-bearing mice. Furthermore, this study aims to determine levels of the primary antioxidants for O −2 and H2O2 dismutation (SOD1, SOD2, SOD3, catalase and GPx), in the skeletal muscle of tumour-bearing cachectic mice (MAC16) compared to tumour-bearing non-cachectic (MAC13) mice.
2 Methods
2.1 Animal model of cancer-induced cachexia
All experimental procedures were carried out with approval from the Victoria University Animal Ethics Committee (AEETH 07/05). Two weight-matched groups of female BALB/c nu/nu mice were maintained under controlled environmental conditions, 12-h light/dark cycle, 21°C ± 2°C, 30% humidity, in conventional cages with ad libitum access to standard chow and water throughout the course of the study. As has previously been described [4], donor mice were used to establish and maintain a tumour line, which consistently produced tumour growth (MAC13) (n = 3) and tumour growth with cachexia (MAC16) (n = 4). Tumour fragments grown and maintained in donor mice were dissected and re-implanted subcutaneously into the flank of recipient mice with frequent monitoring and recording of body weight and tumour size. With cachectic weight loss evident at approximately 9–12 days post implantation, mice were anaesthetised, using pentobarbital sodium (70 mg/kg), for tissue collection before weight loss exceeded 25% of original body weight or before tumour growth exceeded 1,000 mm3. Skeletal muscle tissue was collected, weighed and immediately snap-frozen in liquid nitrogen and stored at −80°C for later use.
2.2 Detection of O −2 by DHE fluorescence staining
Skeletal muscle O −2 was measured using O −2 -sensitive Dihydroethidium (DHE) dye. Cell-permeable DHE reacts with O −2 , converting DHE into ethidium fluorescence. DHE (5 μM) was applied to quadriceps cross-sections (5 μm) and incubated in a light-protected oven at 37°C for 30°min. The sections were washed with PBS to remove excess DHE, and fluorescence was assessed by way of fluorescence microscopy (Axiocam HBO 50/AC, Zeiss, Germany). The muscle was analysed in three sections to obtain measurements from the whole tissue. Ethidium fluorescence density was detected from the whole section with MCID imaging software (Imaging Research Inc, Australia) and expressed as arbitrary units of fluorescence.
2.3 Reverse transcription real-time PCR
RNA was extracted from frozen quadriceps muscle using Tri Reagent (Molecular Research Centre), according to the manufacturer's protocol. Total RNA concentration was determined spectrophotometrically at 260 nm. Prior to reverse transcription (RT), all RNA samples were DNase-treated (Promega), and first-strand cDNA was generated from 1-μg RNA using AMV RT (Promega). Pre-designed TaqMan Gene Expression Assays (Applied Biosystems) were used containing specific primers and probes for the genes of interest. Real-time PCR was performed using Applied Biosystems 7500 detection system and PCRs were performed using TaqMan Gene Expression Master Mix (Applied Biosystems). To compensate for variations in input RNA amounts and efficiency of reverse transcription, GAPDH mRNA was quantified and all results were normalised to these values. Fluorescent emission data were captured and mRNA levels were analysed using the C T value [17].
2.4 Protein electrophoresis and western blotting
Protein was extracted from frozen quadriceps muscle homogenised in ice-cold radio-immunoprecipitation assay buffer containing Tris–HCl (50 mmol/L; pH 7.4), NaCl (150 mmol/L), NP-40 (1%), sodium deoxycholate (0.5%) and SDS (0.1%) and centrifuging at 13,000×g for 15 min at 4°C, to remove insoluble material. The protein concentration was determined by the Bradford method (Bio-Rad) and equal amounts of protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked for 2 h at room temperature in Tris-buffered saline containing Tris–HCl (20 mM; pH 7.6) NaCl (137 mM) and Tween 20 (0.1%) with 5% BSA and probed with primary antibodies for either NOX2 (gp91phox), p40phox, p67phox or GAPDH (1:200; Santa Cruz Biotechnology) overnight at 4°C. Antibody binding was detected using horseradish peroxidase-conjugated secondary antibody (1:50,000; Santa Cruz Biotechnology). The protein bands were detected by SuperSignal West Dura chemiluminescence reagents (Thermo Scientific). The LAS 4000 Imaging System (Fujifilm Life Science, USA) was used to visualize protein bands, and densitometry was performed with MultiGauge software (Fujifilm Life Science, USA). To compensate for variation in protein loading, the relative density of immunoreactive bands were normalised to the density of the corresponding bands for GAPDH.
2.5 Antioxidant enzyme activity and hydrogen peroxide assays
Spectrophotometric assay kits were used to measure SOD (Cayman-706002), catalase (Cayman-707002) and GPx (Cayman- 703102) activity and hydrogen peroxide (Cayman-600050) levels, in muscle homogenates. Frozen muscle pieces (100 mg) were placed in ice-cold HEPES buffer (20 mM) containing EGTA (1 mM), mannitol (210 mM) and sucrose (70 mM) and adjusted to a pH of 7.2 (10 ml/g). Muscle aliquots were homogenised in buffer, using a glass on glass homogeniser, and centrifuged at 1,500×g for 5 min at 4°C to remove insoluble connective tissue. For the detection of SOD1 and SOD2, cytosolic and mitochondrial fractions were separated. The supernatant was centrifuged at 10,000×g for 5 min at 4°C, and the resulting supernatant, containing the cytosolic fraction, was collected for SOD1 enzyme analysis. The remaining pellet containing the mitochondrial fraction was resuspended and homogenised in ice-cold HEPES buffer (20 mM) for SOD2 enzyme analysis. SOD1 and SOD2 activity was measured in cytosolic and mitochondrial muscle fractions, respectively. The amount of enzyme activity and hydrogen peroxide levels were calculated and standardised for protein using the Bradford method (Bio-Rad).
2.6 Statistical analysis
Statistical analysis was performed using SPSS statistical package (version 15.0). Results are expressed as mean ± SEM. Differences were determined by one-way ANOVA with Tukey HSD as posthoc to determine significant differences between groups, and results were considered statistically significant if p values were equal to or <0.05.
3 Results
3.1 Establishment of murine model of cancer and cancer cachexia
Female BALB/c nu/nu mice were implanted with the MAC13 cell line (n = 12) that had previously developed a tumour in the same mouse model at approximately 9–12 days post implantation (n = 3) and where the animal did not lose weight. Another group of female BALB/c nu/nu mice were implanted with the MAC16 cell line (n = 16) that had previously developed a tumour in the same mouse model at approximately 9–12 days post implantation (n = 4) and lost approximately 15–25% of their original body weight were used in this study. Mouse body weight and skeletal muscle weights were recorded at tissue collection and evaluated as a measure of body mass and skeletal muscle cachexia for all mice. Body weight and quadricep weights were significantly less in cachectic (MAC16 tumour-bearing mice) when compared to mice with cancer alone (bearing MAC13 tumours) (p < 0.001; p = 0.003, Table 1). To account for any differences in calorie intake between the groups, food intake was recorded throughout the course of the study and showed no differences between cachectic and cancer mice (Table 1).
3.2 O −2 levels in skeletal muscle of cancer and cancer cachectic mice
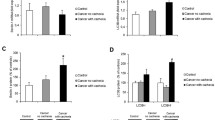
O −2 levels were measured by histological DHE fluorescence in skeletal muscle sections from mice with cancer and cachexia. Skeletal muscle O −2 levels were significantly higher in cachectic mice (9.36 ± 2.67 AU) when compared to mice with cancer (6.47 ± 0.77 AU) alone (p = 0.001, Fig. 1).
O −2 levels in the skeletal muscle of mice with cancer and cancer cachexia. The values represent the mean ± SEM. Statistically significant difference *P = 0.001. a O −2 levels detected by dihydroethidium (DHE) fluorescence expressed as arbitrary units of fluorescence . The values represent the mean ± SEM. Statistically significant difference *P = 0.001. b Histological DHE fluorescence in the skeletal muscle of mice with cancer and cancer cachexia
3.3 mRNA expression of pro-oxidant and antioxidant enzymes in skeletal muscle of cancer and cancer cachectic mice
The mRNA level of the NOX2 and NOX4 enzyme systems previously shown to express in skeletal muscle to generate O −2 were measured in mice with cancer and cancer cachexia. The mRNA of the regulatory and catalytic NOX2 enzyme subunits NOX2, p40phox and p67phox were lower in the skeletal muscle of cachectic mice when compared to mice with cancer alone (p = 0.031; p < 0.001; p = 0.002, Table 2). However, the mRNA expression of the additional NOX enzyme subunits, NOX4, p22phox and p47phox and Rac1 were similar in skeletal muscle from mice bearing with cancer and cancer cachexia (Table 2). The mRNA level of SOD antioxidant enzymes responsible for the dismutation of O −2 were measured in mice with cancer and cancer cachexia. The mRNA level of the SOD1 and SOD2 were significantly lower in the skeletal muscle from cachectic mice when compared to mice with cancer alone (p = 0.007; p < 0.001, Table 2). Similarly, the mRNA level of the H2O2 scavenging antioxidant enzyme GPx was lower in skeletal muscle from cachectic mice compared to mice with cancer alone (p < 0.001, Table 2). However, the antioxidant enzymes SOD3 and catalase had similar mRNA levels in both groups (Table 2).
3.4 Protein levels of NOX2 enzyme subunits in skeletal muscle of cancer and cancer cachectic mice
Changes in the mRNA levels of the key NOX2 enzyme subunits—NOX2, p40phox and p67phox—lead to further analysis of protein expression. The protein expression of the regulatory and catalytic NOX2 enzyme subunits NOX2 and p40phox were lower in cachectic mice when compared to mice with cancer alone (p = 0.021; p < 0.012, Fig. 2). However, the protein expression of p67phox was similar in skeletal muscle from mice in both groups (Fig. 2).
3.5 Antioxidant enzyme activity and hydrogen peroxide levels in skeletal muscle of cancer and cancer cachectic mice
To validate changes in mRNA and protein levels, SOD1, SOD2, catalase and GPx activity levels were measured in skeletal muscle of mice with cancer and cancer cachexia. SOD1 and GPx activities were significantly lower in skeletal muscle from cachectic mice (SOD1 9.9 ± 0.9 U/mg protein, GPx 0.74 ± 0.22 mmol/min/mg protein) when compared to mice with cancer alone (SOD1 11.2 ± 0.8 U/mg protein, GPx 1.48 ± 0.08 mmol/min/mg protein) (p < 0.003, Table 3). However, levels of SOD2 and catalase activity were similar in both groups of (Table 3). H2O2 levels were similar in skeletal muscle from cachectic mice and mice with cancer alone (Table 3).
4 Discussion
Levels of O −2 were increased in cancer-induced skeletal muscle cachexia. Evidence of this was found with increased levels of O −2 detected by DHE fluorescence in the skeletal muscle of cachectic mice, when compared to non-cachectic mice. However, the NOX enzyme systems do not appear to be the source of increased O −2 in cancer-induced skeletal muscle cachexia. In fact, we observed a decrease in the expression of the NOX2 enzyme subunits, NOX2, p40phox and p67phox in cachectic skeletal muscle. Although this study would imply an additional source of O −2 production in cancer-induced skeletal muscle cachexia, what may be of greater importance is the decrease in antioxidant enzyme function, primarily responsible for O −2 dismutation. While this study may be indicative of a lack of compensation by SOD1 and GPx, it may give evidence for their depression, in response to cancer induction and lack of dismutation of even normal cellular O −2 production. The decrease in antioxidant gene expression and activity that function as part of the cellular defence system, for the elimination of O −2 and H2O2, may indicate antioxidant dysfunction in cachectic skeletal muscle, rather than an increase in ROS production.
Cancer induces changes in skeletal muscle that lead to an imbalance in protein synthesis and degradation, resulting in muscle protein loss and function [1]. Interestingly, not all cancer patients develop this secondary condition, and it is this phenomena that has made the development of cancer cachexia relatively undefined [1, 2]. In order to investigate this complex condition, we investigated a model that utilises two similar MAC models, only one of which induces the secondary muscle wasting condition of cancer cachexia. The significant decline in body weight (15–25%) and skeletal muscle mass that was observed in mice bearing the MAC16 tumour, demonstrated the development of cancer-induced skeletal muscle cachexia. While the MAC16 cancer cachectic model is well-established in NMRI mice [4], this study mimics the cachectic condition in MAC16 tumour-bearing nude mice. This model of cancer and cancer cachexia establishes a direct comparison between a cancer control and cancer cachectic mouse model. Contrasting studies have implicated the importance of calorie restriction in the development of cancer cachexia; however, our study, like others [18], did not observe any change in food intake from cachectic mice. This study therefore suggests a more complex catabolic-mediated response to protein degeneration, in the development of cancer cachexia.
The physical changes in skeletal muscle were mirrored by significant cellular oxidative changes in response to MAC16 induction and cachectic development. In particular, the significant increase in O −2 levels observed in cachectic skeletal muscle indicates that O −2 is implicated in the cancer cachectic condition. Previous studies have proposed a central mediating role for ROS in the development of cancer cachexia [5, 18]; however, the source of ROS in this process remains to be elucidated. The knowledge that the NOX enzyme system functions primarily to produce O −2 [19], recognises NOX-generated O −2 as more than a by-product of cellular metabolism, but rather a product of a regulated response to stimuli for a physiological purpose. This function of NOX makes this enzyme system the potential source of O −2 , vulnerable to changes associated with the cancer condition and development of cachexia. To our knowledge, this study is the first to investigate the NOX enzymes in cancer cachectic skeletal muscle. Interestingly, the NOX2 enzyme has consistently been shown to increase in degenerative conditions [19]; however, this study does not support an increase in the NOX2 enzyme in cancer cachexia.
It is evident, however, from this study and others, that the absence of a functioning antioxidant enzyme system to dismutate O −2 is present in cancer cachectic skeletal muscle [20]. Cellular oxidative stress has been implicated in skeletal muscle wasting conditions with marked attenuation following antioxidant induction [21]. Despite the increase in O -2 in cachectic skeletal muscle that is indicative of a need for SOD activity, we observed a depression in SOD1 activity levels. Furthermore, our SOD gene expression data would appear to suggest that the inability for SOD to compensate for the increase in O −2 is regulated at the gene level. Alterations in this important protective system can lead to oxidative imbalance, and induce critical changes to cellular structure and function [20]. Antioxidant enzymes are valuable indicators of ROS production as well as changes in cellular redox signalling and evidence of cell functionality [13]. The consequences of SOD antioxidant enzyme modifications have been demonstrated in studies investigating SOD knockout models. Muller et al. [22] demonstrated an age-dependent loss of muscle mass in mice lacking SOD1, as well as a significant decrease in their average life span. Furthermore, Sun et al. [23] investigated the effects of SOD1 and SOD2 overexpression in Drosophila that demonstrated a decrease in cumulative oxidative damage and increased metabolic potential, with an increased life span by up to 37% and 75%, respectively. These models demonstrate a crucial role for SOD1 shown through its significant contribution to oxidative damage and muscle degeneration.
It is also not surprising that with a decline in O −2 dismutation by SOD, to convert O −2 to H2O2, the gene expression and activity of the H2O2 scavenging antioxidant enzyme GPx would also decrease. Consequently, these important cellular systems would indeed contribute to significant changes in redox-sensitive signalling pathways in cancer cachexia. SOD, not only has a crucial role in eliminating O −2 accumulation, but also plays an important role in intracellular redox-sensitive signalling and regulation of oxidative systems [13]. As ROS have been described as important mediators of redox-sensitive intracellular signalling, so too are the antioxidants that regulate them. It is well-known that with the generation of intracellular O −2 , SOD1 functions to dismute O −2 to H2O2 [14]. H2O2 in particular, has been shown to be involved in numerous signalling cascades, and therefore, its cellular regulation, via SOD, has the potential to influence a number of important cellular pathways [24]. The changes in SOD1 function would indeed have critical consequences to cellular function that is most certainly redox-related.
A circulatory protein, PIF was first described as a causative agent in cachexia when it was discovered in mice expressing the MAC16 tumour [25]. As this glycoprotein was initially discovered in the sera of mice bearing the cachectic MAC16 tumour, but not in the sera of the mice bearing the non-cachectic MAC13 tumours, it was regarded as an important factor in the development of cancer cachexia [25]. The accelerated loss of skeletal muscle protein in cachexia has been attributed to Ub-proteasome pathway activation [15, 26], and PIF has been shown to induce this pathway of skeletal muscle atrophy [26]. Although the PIF/Ub pathway indeed plays an important role in the development of cancer cachexia [15], a number of additional mechanisms are most likely involved [27]. Preproteasomal mechanisms, mediators, receptor binding, signalling pathways and activation of specific transcription factors are all important considerations [3] and ROS have been implicated in these cellular processes.
Collectively, the results of this study suggest influential signalling involving O −2 and antioxidant enzymes in cancer cachectic skeletal muscle. However, the signalling pathway(s) leading to the decrease in the NOX2 subunits and antioxidant enzyme expression, together with decreased antioxidant enzyme activity in cachectic skeletal muscle remains undefined. In response to ROS, cells activate the expression of a number of genes via transcription factor regulation, leading to modifications in the gene expression of important proteins, including antioxidant enzymes [13] and those involved in muscle protein synthesis and regeneration [28]. Furthermore, it is possible to speculate that the downregulation of the NOX2 enzyme system is a compensatory response to O −2 accumulation, induced primarily by SOD and GPx antioxidant system dysfunction and is therefore a regulated response to O −2 build-up in the cellular system. However, with the knowledge that ROS can cause damage to cellular proteins [29], it is possible that the decrease in the NOX2 enzyme subunits and antioxidant enzymes is a result of protein oxidation. With this in mind, it would be important to investigate these oxidative systems and the oxidative status of cachectic skeletal muscle during the progression of the disease.
5 Conclusion
It is evident from cachectic studies that show the presence of oxidised proteins and attenuation following antioxidant administration that ROS play an important role in the development of cachexia. Although the exact mechanisms are poorly defined, experimental research in the NOX enzyme systems have indicated a number of important roles for ROS, in addition to direct oxidative tissue damage, for its involvement in redox-sensitive signalling pathways. While studies have implicated the involvement of ROS in the pathogenesis of cachexia and have suggested a role for NOX, this study suggests that the increase in O −2 in cachectic skeletal muscle is a result of antioxidant dysfunction. However, what remains unclear is whether this result is a regulated response in the cellular system or an important contributor to the changes observed in skeletal muscle physiology. Furthermore, with the well-established role for the Ub-proteasome pathway in cancer cachexia, the increase in O −2 levels in our study provides evidence of a signalling role for O −2 in this pathway of skeletal muscle atrophy in cancer cachexia. While these results, along with the additional findings of this study indeed demonstrate complex changes in cachectic skeletal muscle, this multifactoral condition coupled with a multifunctional system, further demonstrates the complexity of skeletal muscle response(s) to cancer induction. It is therefore important to understand further the role that these oxidative and antioxidative systems play in the skeletal muscle system and development of cancer cachexia.
References
Giordano A, Calvani M, Petillo O, Carteni M, Melone MR, Peluso G. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem. 2003;90:170–86.
Tijerina AJ. The biochemical basis of metabolism in cancer cachexia. Dimens Crit Care Nurs. 2004;23:237–43.
Hasselgren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun. 2002;290:1–10.
Hussey HJ, Tisdale MJ. Effect of a cachectic factor on carbohydrate metabolism and attenuation by eicosapentaenoic acid. Br J Cancer. 1999;80:1231–5.
Barreiro E, de la Puente B, Busquets S, Lopez-Soriano FJ, Gea J, Argiles JM. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005;579:1646–52.
Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem. 2007;282:7087–97.
Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–73.
Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–16.
Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1165–70.
Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–40.
Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306.
Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem. 2006;281:26473–82.
Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–93.
Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49.
Smith HJ, Tisdale MJ. Signal transduction pathways involved in proteolysis-inducing factor induced proteasome expression in murine myotubes. Br J Cancer. 2003;89:1783–8.
Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal. 2007;19:1797–806.
Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204.
Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. 2000;16:1015–8.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed Pharmacother. 2005;59:139–42.
Hussey HJ, Bibby MC, Tisdale MJ. Novel anti-tumour activity of 2,3,5-trimethyl-6-(3-pyridylmethyl)-1,4- benzoquinone (CV-6504) against established murine adenocarcinomas (MAC). Br J Cancer. 1996;73:1187–92.
Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004.
Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–72.
True AL, Rahman A, Malik AB. Activation of NF-kappaB induced by H(2)O(2) and TNF-alpha and its effects on ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L302–11.
Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature. 1996;379:739–42.
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51.
Tisdale MJ. Tumor-host interactions. J Cell Biochem. 2004;93:871–7.
Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67.
Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–8.
von Haehling S, Morley J, Coats A, SD A. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.
Acknowledgements
This research was supported by Deakin University and Victoria University. Melanie Sullivan-Gunn was the recipient of a Victoria University postgraduate scholarship. The authors would like to thank the staff of the Deakin University Building Lp Animal House for their help and support with the animal study. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [30].
Conflicts of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sullivan-Gunn, M.J., Campbell-O’Sullivan, S.P., Tisdale, M.J. et al. Decreased NADPH oxidase expression and antioxidant activity in cachectic skeletal muscle. J Cachexia Sarcopenia Muscle 2, 181–188 (2011). https://doi.org/10.1007/s13539-011-0037-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-011-0037-3