Abstract
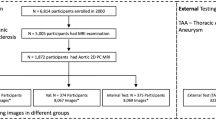
Diagnosis of ascending thoracic aortic aneurysm (ATAA) is based on the measurement of the maximum aortic diameter, but size is not a good predictor of the risk of adverse events. There is growing interest in the development of novel image-derived risk strategies to improve patient risk management towards a highly individualized level. In this study, the feasibility and efficacy of deep learning for the automatic segmentation of ATAAs was investigated using UNet, ENet, and ERFNet techniques. Specifically, CT angiography done on 72 patients with ATAAs and different valve morphology (i.e., tricuspid aortic valve, TAV, and bicuspid aortic valve, BAV) were semi-automatically segmented with Mimics software (Materialize NV, Leuven, Belgium), and then used for training of the tested deep learning models. The segmentation performance in terms of accuracy and time inference were compared using several parameters. All deep learning models reported a dice score higher than 88%, suggesting a good agreement between predicted and manual ATAA segmentation. We found that the ENet and UNet are more accurate than ERFNet, with the ENet much faster than UNet. This study demonstrated that deep learning models can rapidly segment and quantify the 3D geometry of ATAAs with high accuracy, thereby facilitating the expansion into clinical workflow of personalized approach to the management of patients with ATAAs.






Similar content being viewed by others
References
Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73(1):17–28.
Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370(20):1920–9. https://doi.org/10.1056/NEJMra1207059.
Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–12. https://doi.org/10.1001/jama.2011.1286.
Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’Gara PT, Evangelista A, et al. Aortic diameter > = 55 cm is not a good predictor of type A aortic dissection—observations from the international registry of acute aortic dissection (IRAD). Circulation. 2007;116(10):1120–7. https://doi.org/10.1161/circulationaha.107.702720.
Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156(2):e41–74. https://doi.org/10.1016/j.jtcvs.2018.02.115.
Mohammadi S, Mohammadi M, Dehlaghi V, Ahmadi A. Automatic segmentation, detection, and diagnosis of abdominal aortic aneurysm (AAA) using convolutional neural networks and hough circles algorithm. Cardiovasc Eng Technol. 2019;10(3):490–9. https://doi.org/10.1007/s13239-019-00421-6.
Lopez-Linares K, Aranjuelo N, Kabongo L, Maclair G, Lete N, Ceresa M, et al. Fully automatic detection and segmentation of abdominal aortic thrombus in post-operative CTA images using Deep Convolutional Neural Networks. Med Image Anal. 2018;46:202–14. https://doi.org/10.1016/j.media.2018.03.010.
Baskaran L, Al’Aref SJ, Maliakal G, Lee BC, Xu Z, Choi JW, et al. Automatic segmentation of multiple cardiovascular structures from cardiac computed tomography angiography images using deep learning. PLoS ONE. 2020;15(5):e0232573. https://doi.org/10.1371/journal.pone.0232573.
Baskaran L, Maliakal G, Al’Aref SJ, Singh G, Xu Z, Michalak K, et al. Identification and quantification of cardiovascular structures from CCTA: an end-to-end, rapid, pixel-wise, deep-learning method. JACC Cardiovasc Imaging. 2020;13(5):1163–71. https://doi.org/10.1016/j.jcmg.2019.08.025.
Dahiya N, Yezzi A, Piccinelli M, Garcia E. Integrated 3D Anatomical Model for Automatic Myocardial Segmentation in Cardiac CT Imagery. Comput Methods Biomech Biomed Eng Imaging Vis. 2019;7(5–6):690–706. https://doi.org/10.1080/21681163.2019.1583607.
Zaharchuk G, Gong E, Wintermark M, Rubin D, Langlotz CP. Deep learning in neuroradiology. AJNR Am J Neuroradiol. 2018;39(10):1776–84. https://doi.org/10.3174/ajnr.A5543.
Ronneberger O, Fischer P, Broz T. U-net: convolutional networks for biomedical image segmentation. In: Lecture notes in computer science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 2015.
Paszke A, Chaurasia A, Kim S, Culurciello E. ENet: A deep neural network architecture for real-time semantic segmentation. ArXiv. 2016;1606.02147.
Romera E, Alvarez JM, Bergasa LM, Arroyo R. ERFNet: efficient residual factorized ConvNet for real-time semantic segmentation. IEEE Trans Intell Transp Syst. 2018;19(1):263–72.
Pasta S, Gentile G, Raffa GM, Bellavia D, Chiarello G, Liotta R, et al. In silico shear and intramural stresses are linked to aortic valve morphology in dilated ascending aorta. Eur J Vasc Endovasc Surg. 2017;S1078–5884(17):30331–3.
Rinaudo A, Raffa GM, Scardulla F, Pilato M, Scardulla C, Pasta S. Biomechanical implications of excessive endograft protrusion into the aortic arch after thoracic endovascular repair. Comput Biol Med. 2015;66:235–41. https://doi.org/10.1016/j.compbiomed.2015.09.011.
Pasta S, Gentile G, Raffa GM, Scardulla F, Bellavia D, Luca A, et al. Three-dimensional parametric modeling of bicuspid aortopathy and comparison with computational flow predictions. Artif Organs. 2017;41(9):E92–E102. https://doi.org/10.1111/aor.12866.
Srivastava N, Hinto G, Krizhevsky A, Sutskever I, Salakhutdinov R. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res. 2014;15(56):1929–58.
Masko D, Hensman P. The impact of imbalanced training data for convolutional neural networks. 2015.
Salehi SS, Erdogmus D, Gholipour A. Tversky loss function for image segmentation using 3D fully convolutional deep networks. In: Lecture notes in computer science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics). 2017.
Kingma D, Ba J. A method for stochastic optimization. In: Proceedings of the 3rd international conference on learning representations 2015.
Comelli A, Stefano A, Russo G, Sabini MG, Ippolito M, Bignardi S, et al. A smart and operator independent system to delineate tumours in Positron Emission Tomography scans. Comput Biol Med. 2018;102:1–15. https://doi.org/10.1016/j.compbiomed.2018.09.002.
Comelli A, Bignardi S, Stefano A, Russo G, Sabini MG, Ippolito M, et al. Development of a new fully three-dimensional methodology for tumours delineation in functional images. Comput Biol Med. 2020;120:103701. https://doi.org/10.1016/j.compbiomed.2020.103701.
Maffei E, Messalli G, Martini C, Nieman K, Catalano O, Rossi A, et al. Left and right ventricle assessment with Cardiac CT: validation study vs. Cardiac MR. Eur Radiol. 2012;22(5):1041–9. https://doi.org/10.1007/s00330-011-2345-6.
Ecabert O, Peters J, Walker MJ, Ivanc T, Lorenz C, von Berg J, et al. Segmentation of the heart and great vessels in CT images using a model-based adaptation framework. Med Image Anal. 2011;15(6):863–76. https://doi.org/10.1016/j.media.2011.06.004.
Roth HR, Oda H, Zhou X, Shimizu N, Yang Y, Hayashi Y, et al. An application of cascaded 3D fully convolutional networks for medical image segmentation. Comput Med Imaging Graph Off J Comput Med Imaging Soc. 2018;66:90–9. https://doi.org/10.1016/j.compmedimag.2018.03.001.
Comelli A, Stefano A, Bignardi S, Russo G, Sabini MG, Ippolito M, et al. Active contour algorithm with discriminant analysis for delineating tumors in positron emission tomography. Artif Intell Med. 2019;94:67–78. https://doi.org/10.1016/j.artmed.2019.01.002.
Comelli A, Stefano A, Russo G, Bignardi S, Sabini MG, Petrucci G, et al. K-nearest neighbor driving active contours to delineate biological tumor volumes. Eng Appl Artif Intell. 2019;81:133–44.
Della Corte A, Bancone C, Dialetto G, Covino FE, Manduca S, Montibello MV, et al. The ascending aorta with bicuspid aortic valve: a phenotypic classification with potential prognostic significance. Eur J Cardiothorac Surg. 2014;46(2):240–7. https://doi.org/10.1093/ejcts/ezt621(discussion 7).
Cosentino F, Scardulla F, D’Acquisto L, Agnese V, Gentile G, Raffa G, et al. Computational modeling of bicuspid aortopathy: towards personalized risk strategies. J Mol Cell Cardiol. 2019;131:122–31. https://doi.org/10.1016/j.yjmcc.2019.04.026.
Bieging ET, Frydrychowicz A, Wentland A, Landgraf BR, Johnson KM, Wieben O, et al. In vivo three-dimensional MR wall shear stress estimation in ascending aortic dilatation. J Magn Reson Imaging. 2011;33(3):589–97. https://doi.org/10.1002/Jmri.22485.
Farzaneh S, Trabelsi O, Avril S. Inverse identification of local stiffness across ascending thoracic aortic aneurysms. Biomech Model Mechanobiol. 2018. https://doi.org/10.1007/s10237-018-1073-0.
Gallo A, Agnese V, Coronnello C, Raffa GM, Bellavia D, Conaldi PG, et al. On the prospect of serum exosomal miRNA profiling and protein biomarkers for the diagnosis of ascending aortic dilatation in patients with bicuspid and tricuspid aortic valve. Int J Cardiol. 2018;15;273:230–236. https://doi.org/10.1016/j.ijcard.2018.10.005.
Pasta S, Agnese V, Gallo A, Cosentino F, Di Giuseppe M, Gentile G, et al. Shear stress and aortic strain associations with biomarkers of ascending thoracic aortic aneurysm. Ann Thorac Surg. 2020;110(5):1595–1604. https://doi.org/10.1016/j.athoracsur.2020.03.017.
Liang L, Liu M, Martin C, Elefteriades JA, Sun W. A machine learning approach to investigate the relationship between shape features and numerically predicted risk of ascending aortic aneurysm. Biomech Model Mechanobiol. 2017;16(5):1519–33. https://doi.org/10.1007/s10237-017-0903-9.
Cosentino F, Raffa GM, Gentile G, Agnese V, Bellavia D, Pilato M, et al. Statistical shape analysis of ascending thoracic aortic aneurysm: correlation between shape and biomechanical descriptors. J Personal Med. 2020. https://doi.org/10.3390/jpm10020028.
Pasta S, Agnese V, Di Giuseppe M, Gentile G, Raffa GM, Bellavia D, et al. In vivo strain analysis of dilated ascending thoracic aorta by ECG-Gated CT angiographic imaging. Ann Biomed Eng. 2017;45(12):2911–2920. https://doi.org/10.1007/s10439-017-1915-4.
Acknowledgements
This work was partially supported by a grant (W911NF-18-1-0281) from USA Army Research Office (ARO) to Anthony Yezzi, by a grant (R01-HL-143350) from National Institute of Health (NIH) to Anthony Yezzi, and by a grant (GR-2011-02348129) from the Italian Ministry of Health to Salvatore Pasta.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Albert Comelli declares that he has no conflict of interest. Navdeep Dahiya declares that he has no conflict of interest. Alessandro Stefano declares that he has no conflict of interest. Viviana Benfante declares that she has no conflict of interest. Giovanni Gentile declares that he has no conflict of interest. Valentina Agnese declares that she has no conflict of interest. Giuseppe M Raffa declares that he has no conflict of interest. Michele Pilato declares that he has no conflict of interest. Anthony Yezzi declares that he has no conflict of interest. Giovanni Petrucci declares that he has no conflict of interest. Salvatore Pasta declares that he has no conflict of interest.
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Comelli, A., Dahiya, N., Stefano, A. et al. Deep learning approach for the segmentation of aneurysmal ascending aorta. Biomed. Eng. Lett. 11, 15–24 (2021). https://doi.org/10.1007/s13534-020-00179-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-020-00179-0