Abstract
Demographic studies of mortality often emphasize the two ends of the lifespan, focusing on the declining hazard after birth or the increasing risk of death at older ages. We call attention to the intervening phase, when humans are least vulnerable to the force of mortality, and consider its features in both evolutionary and historical perspectives. We define this quiescent phase (Q-phase) formally, estimate its bounds using life tables for Swedish cohorts born between 1800 and 1920, and describe changes in the morphology of the Q-phase. We show that for cohorts aging during Sweden’s demographic and epidemiological transitions, the Q-phase became longer and more pronounced, reflecting the retreat of infections and maternal mortality as key causes of death. These changes revealed an underlying hazard trajectory that remains relatively low and constant during the prime ages for reproduction and investment in both personal capital and relationships with others. Our characterization of the Q-phase highlights it as a unique, dynamic, and historically contingent cohort feature, whose increased visibility was made possible by the rapid pace of survival improvements in the nineteenth and twentieth centuries. This visibility may be reduced or sustained under subsequent demographic regimes.






Similar content being viewed by others
References
Austad, S. N., & Partridge, L. (1997). Why we age: What science is discovering about the body’s journey through life. New York, NY: Wiley & Sons.
Barker, D. J. P. (2004). The developmental origins of well-being. Philosophical Transactions of the Royal Society of London: Series B (Biological Sciences), 359, 1359–1366.
Bebbington, M., Lai, C.-D., & Zitikis, R. (2006). Useful periods for lifetime distributions with bathtub shaped hazard rate functions. IEEE Transactions on Reliability, 55(2), 245–251.
Beltrán-Sánchez, H., Crimmins, E. M., & Finch, C. E. (2012). Early cohort mortality predicts the rate of aging in the cohort: A historical analysis. Journal of Developmental Origins of Health and Disease, 3, 380–386.
Bengtsson, T. (2004). Life under pressure: Mortality and living standards in Europe and Asia, 1700–1900. Cambridge, MA: MIT Press.
Bengtsson, T., & Dribe, M. (2011). The late emergence of socioeconomic mortality differentials: A micro-level study of adult mortality in southern Sweden 1815–1968. Explorations in Economic History, 48, 389–400.
Bengtsson, T., & Lindström, M. (2000). Childhood misery and disease in later life: The effects on mortality in old age of hazards experienced in early life, southern Sweden, 1760–1894. Population Studies, 54, 263–277.
Black, R. E., Cousens, S., Johnson, H. L., Lawn, J. E., Rudan, I., Bassani, D. G., . . . Mathers, C. (2010). Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet, 375, 1969–1987.
Bongaarts, J., & Feeney, G. (2002). How long do we live? Population and Development Review, 28, 13–29.
Booth, H., & Tickle, L. (2008). Mortality modelling and forecasting: A review of methods. Annals of Actuarial Science, 3, 3–43.
Bribiescas, R. G. (2001). Reproductive ecology and life history of the human male. American Journal of Physical Anthropology, 116(S33), 148–176.
Carnes, B. A., & Olshansky, S. J. (1997). A biologically motivated partitioning of mortality. Experimental Gerontology, 32, 615–631.
Caughley, G. (1966). Mortality patterns in mammals. Ecology, 47, 906–918.
Charlesworth, B. (1994). Evolution in age-structured populations (2nd ed.). Cambridge, UK: Cambridge University Press.
Chu, C. Y. C., Chien, H.-K., & Lee, R. D. (2008). Explaining the optimality of U-shaped age-specific mortality. Theoretical Population Biology, 73, 171–180.
Crimmins, E. M., & Finch, C. E. (2006). Infection, inflammation, height, and longevity. Proceedings of the National Academy of Sciences, 103, 498–503.
Engelman, M., Caswell, H., & Agree, E. M. (2014). Why do lifespan variability trends for the young and old diverge? A perturbation analysis. Demographic Research, 30(article 48), 1367–1396. doi:10.4054/DemRes.2014.30.48
Finch, C. E. (2012). Evolution of the human lifespan, past, present, and future: Phases in the evolution of human life expectancy in relation to the inflammatory load. Proceedings of the American Philosophical Society, 156, 9–44.
Finch, C. E., Pike, M. C., & Witten, M. (1990). Slow mortality rate accelerations during aging in some animals approximate that of humans. Science, 249, 902–905.
Fridlizius, G. (1989). The deformation of cohorts: Nineteenth century mortality decline in a generational perspective. Scandinavian Economic History Review, 37(3), 3–17.
Gage, T. B. (1998). The comparative demography of primates: With some comments on the evolution of life histories. Annual Review of Anthropology, 27, 197–221.
Gage, T. B., & Dyke, B. (1986). Parameterizing abridged mortality tables: The Siler three-component hazard model. Human Biology, 58, 275–291.
Gage, T. B., & Mode, C. J. (1993). Some laws of mortality: How well do they fit? Human Biology, 65, 445–461.
Gavrilov, L. A., & Gavrilova, N. S. (2001). The reliability theory of aging and longevity. Journal of Theoretical Biology, 213, 527–545.
Goldstein, J. R. (2011). A secular trend toward earlier male sexual maturity: Evidence from shifting ages of male young adult mortality. PloS One, 6(8), e14826. doi:10.1371/journal.pone.0014826
Goldstein, J. R., & Wachter, K. W. (2006). Relationships between period and cohort life expectancy: Gaps and lags. Population Studies, 60, 257–269.
Gompertz, B. (1825). On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society of London, 115, 513–583.
Guillot, M. (2003). The cross-sectional average length of life (CAL): A cross-sectional mortality measure that reflects the experience of cohorts. Population Studies, 57, 41–54.
Guillot, M. (2011). Period versus cohort life expectancy. In R. G. Rogers & E. M. Crimmins (Eds.), International handbook of adult mortality (pp. 533–549). Dordrecht, The Netherlands: Springer.
Gurven, M., & Kaplan, H. (2007). Longevity among hunter-gatherers: A cross-cultural examination. Population and Development Review, 33, 321–365.
Hamilton, W. D. (1966). The moulding of senescence by natural selection. Journal of Theoretical Biology, 12, 12–45.
Hawkes, K., O’Connell, J. F., Jones, N. G. B., Alvarez, H., & Charnov, E. L. (1998). Grandmothering, menopause, and the evolution of human life histories. Proceedings of the National Academy of Sciences, 95, 1336–1339.
Heligman, L., & Pollard, J. H. (1980). The age pattern of mortality. Journal of the Institute of Actuaries, 107, 49–80.
Human Mortality Database. (2015). Berkeley: University of California, Berkeley; and Rostock, Germany: Max Planck Institute for Demographic Research. Available from www.mortality.org
Jones, H. B. (1961). Mechanism of aging suggested from study of altered death risks. In J. Neyman (Ed.), Proceedings of the Berkeley Symposium on Mathematical Statistics and Probability (Vol. 4, pp. 267–292). Berkeley: University of California Press.
Kermack, W. O., McKendrick, A. G., & McKinlay, P. L. (2001). Death-rates in Great Britain and Sweden. Some general regularities and their significance. International Journal of Epidemiology, 30, 678–683.
Kruger, D. J., & Nesse, R. M. (2006). An evolutionary life-history framework for understanding sex differences in human mortality rates. Human Nature, 17, 74–97.
Lee, R. D. (2003). Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proceedings of the National Academy of Sciences, 100, 9637–9642.
Levitis, D. A. (2011). Before senescence: The evolutionary demography of ontogenesis. Proceedings of the Royal Society B: Biological Sciences, 278, 801–809.
Livi-Bacci, M. (2012). A concise history of world population (5th ed.). West Sussex, UK: John Wiley & Sons.
Loudon, I. (1992). Death in childbirth: An international study of maternal care and maternal mortality 1800–1950. Oxford, UK: Clarendon Press.
Makeham, W. M. (1889). On the further development of Gompertz’s law. Journal of the Institute of Actuaries, 28, 152–159.
Marmot, M. G., Bosma, H., Hemingway, H., Brunner, E., & Stansfeld, S. (1997). Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet, 350, 235–239.
Medawar, P. B. (1952). An unsolved problem of biology. London, UK: H.K. Lewis.
Medawar, P. B. (1955). The definition and measurement of senescence. In G. E. W. Wolstenholme & M. P. Cameron (Eds.), Ciba Foundation Symposium: Colloquia on ageing - General aspects (Vol. 1, pp. 4–15). Boston, MA: Little Brown & Company.
Medawar, P. B. (1957). Uniqueness of the individual. London, UK: Methuen.
Nash, J. C., & Varadhan, R. (2011). Unifying optimization algorithms to aid software system users: Optimx for R. Journal of Statistical Software, 43(9), 1–14.
Olshansky, S. J., & Carnes, B. A. (1997). Ever since Gompertz. Demography, 34, 1–15.
Ouellette, N., Barbieri, M., & Wilmoth, J. R. (2014). Period-based mortality change: Turning points in trends since 1950. Population and Development Review, 40, 77–106.
Partridge, L. (2010). The new biology of ageing. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 365, 147–154.
Pearl, R., & Miner, J. R. (1935). Experimental studies on the duration of life. XIV. The comparative mortality of certain lower organisms. Quarterly Review of Biology, 10, 60–79.
Riley, J. C. (2001). Rising life expectancy: A global history. Cambridge, UK: Cambridge University Press.
Robson, A. J., & Kaplan, H. S. (2003). The evolution of human life expectancy and intelligence in hunter-gatherer economies. American Economic Review, 93, 150–169.
Ryder, N. B. (1964). The process of demographic translation. Demography, 1, 74–82.
Schön, L., & Schubert, K. (2010). Sweden’s road to modernity: An economic history. Stockholm, Sweden: SNS Förlag.
Siler, W. (1979). A competing-risk model for animal mortality. Ecology, 60, 750–757.
Thiele, T. B., & Sprague, T. B. (1871). On a mathematical formula to express the rate of mortality throughout the whole of life, tested by a series of observations made use of by the Danish life insurance company of 1871. Journal of the Institute of Actuaries, 16, 313–329.
Vaupel, J. W. (2003). Post-Darwinian longevity. Population and Development Review, 29, 258–269.
Vaupel, J. W., Carey, J. R., Christensen, K., Johnson, T. E., Yashin, A. I., Holm, N. V., . . . Curtsinger, J. W. (1998). Biodemographic trajectories of longevity. Science, 280, 855–860.
Vaupel, J. W., & Yashin, A. I. (1985). Heterogeneity’s ruses: Some surprising effects of selection on population dynamics. American Statistician, 39, 176–185.
Wachter, K. (2003). Hazard curves and lifespan prospects. Population and Development Review, 29(Suppl.), 270–291.
Wachter, K., Steinsaltz, D., & Evans, S. N. (2014). Evolutionary shaping of demographic schedules. Proceedings of the National Academy of Sciences, 111, 10846–10853.
White, H. (1980). A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica, 48, 817–838.
Wilmoth, J. R. (1989). Variation in vital rates by age, period, and cohort. Sociological Methodology, 20, 295–335.
Zeileis, A. (2006). Object-oriented computation of sandwich estimators (Research Report Series, No. 37). Vienna, Austria: WU Vienna University of Economics and Business, Department of Statistics and Mathematics. Retrieved from http://epub.wu.ac.at/1644/
Acknowledgments
Michal Engelman began work on this project while supported by a postdoctoral fellowship in the Epidemiology and Biostatistics of Aging at the Johns Hopkins Center on Aging and Health (NIA T32AG000247). She is now supported by the Center for Demography and Ecology (NICHD R24 HD047873) and Center for Demography of Health and Aging (NIA P30 AG17266) at the University of Wisconsin–Madison. Christopher L. Seplaki was supported by Mentored Research Scientist Development Award number K01AG031332 from the National Institute on Aging. Ravi Varadhan was a Brookdale Leadership in Aging Fellow. This work was also funded in part by the Older Americans’ Independence Center (OAIC) at the Johns Hopkins University. Previous versions of this article were presented at meetings of the Population Association of America and at the Berkeley Formal Demography Workshop. We thank John Wilmoth, Ron Lee, Joshua Goldstein, and Joshua Garoon for helpful comments and discussion. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Appendix: Parameter Estimation Methods
Appendix: Parameter Estimation Methods
Although the hazard function and the survival probability function are mathematically equivalent—that is, there is a strict one-to-one correspondence between them—hazard functions are typically difficult to estimate from empirical data because they tend to be very noisy. Thus, in most survival analysis contexts, the analyst works with the survival probability model that corresponds to the hazard in order to estimate the parameters by maximizing the likelihood of the observed events. However, life tables are unique in containing relatively complete information (in terms of person-years of exposure and number of events) on the event history for the population of interest. This makes the direct computation and estimation of continuous hazard functions more tractable relative to other analytic contexts.
We can obtain the Siler model parameters in two different ways: (1) directly fitting the Siler hazard to the empirically observed hazards from life tables via nonlinear least squares (NLS) estimation, and (2) maximizing the likelihood function for the probability of death, an approach known as maximum likelihood estimation (MLE).
In the direct approach, we obtain the parameters by minimizing the sum of squared deviations of model-predicted and observed log of survival probability: that is, we minimize (Σx n [log(q(x n )) – log(1 – e μ( x n ))]2). This is the NLS approach. In the second approach, we write the binomial likelihood that corresponds to d(x) deaths of a total of l(x) individuals, where the probability of a single death is q(x) = 1 – exp(–μ(x)). This likelihood is a function of the parameters involved in μ(x). We maximize this likelihood for the observed life table data (d(x n ) and l(x n )) to obtain the Siler model parameters. This is MLE.
The two approaches for parameter estimation are asymptotically equivalent. Empirically, however, the parameter estimates and standard errors generated by each approach might differ slightly because of the finite sample size (especially for the oldest ages) and the discretization of continuous age into one-year intervals. Although maximum likelihood may intuitively be expected to yield parameter estimates with the best model fit based on the maximum likelihood estimator’s asymptotic properties, in specific (finite) samples representing whole populations where accounting for sampling is not a concern, the quality of parameter estimates from a directly fitted hazard may in fact be superior.
optimx (Nash and Varadhan 2011) is a unique tool that integrates several optimization algorithms available in R. More than a dozen algorithms including several Newton-type, gradient, and derivative-free algorithms are implemented under one simple call. optimx organizes the results of multiple algorithms according to the values of the objective function. The results are provided in a manner that facilitates the comparison of the performance of different algorithms in terms of objective function values, computational effort, and the quality of solution (i.e., whether the solution satisfies first- and second-order Kuhn-Karrush-Tucker conditions for local optimum). We employed optimx for both the NLS and MLE approaches.
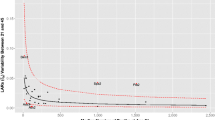
We found that the NLS estimation approach is slightly better than the MLE approach in terms of better convergence of iterative algorithms for parameter estimation. Although the MLE estimates provided a better fit of the survival probability, the NLS approach provides a better fit to the observed mortality hazard (see Fig. 7), as determined by the closeness of the fitted values to the empirically observed values. Results and figure references in the main text therefore rely on the NLS estimates.
The empirical mortality hazard trajectory (left) and survival probability (right) for the cohort of Swedish females born in 1889, along with trajectories fitted using maximum likelihood (MLE; blue dots) and nonlinear least squares (NLS; red dashes) methods. The NLS method provides a better fit for the hazard, but the MLE provides a better fit for the survival curve
Rights and permissions
About this article
Cite this article
Engelman, M., Seplaki, C.L. & Varadhan, R. A Quiescent Phase in Human Mortality? Exploring the Ages of Least Vulnerability. Demography 54, 1097–1118 (2017). https://doi.org/10.1007/s13524-017-0569-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13524-017-0569-z