Abstract
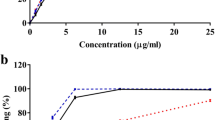
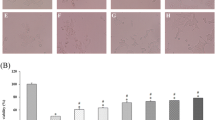
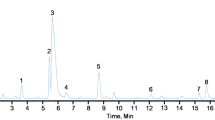
Strychnos henningsii (SH) is a plant commonly used in southern African traditional medicine for the management of diabetes mellitus. Previous in vivo studies showed that a stem bark extract improves glycemic control in a diabetic animal model. Meanwhile, the mechanism of action has not been elucidated. The present study therefore investigated various in vitro models known to target glucose homeostasis and its direct complications. The plant extract was found to stimulate both basal and insulin stimulated glucose uptake in differentiated 3T3-L1 cells but not in Chang liver cells. The effect on 3T3-L1 cells appears independent of PPARγ as the extract did not stimulate adipogenesis. Although, SH extract was inhibitory toward intestinal alpha glucosidase, the physiological relevance is doubtful based on the recommended dosages. The extract strongly inhibited protein glycation which, at least in part, may be explained by the antioxidant and phenolic content of this plant. Cytotoxicity in Chang liver cells yielded an IC50 value of 130.0 μg/mL raising concern that continual exposure to this herbal remedy may initiate hepatotoxicity. The finding from this study suggests that SH extract may attenuate hyperglycemia through enhanced peripheral tissue glucose utilization.





Similar content being viewed by others
References
Jakus V, Hrnciarova M, Carsky J, Krahulec B, Rietbrock N. Incubation of non enzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity. Life Sci. 1999;65:1991–3.
Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–62.
Surekha RH, Srikanh BMV, Jharna P, Ramachandra RV, Dayasagar RV, Jyothy A. Oxidative stress and total antioxidant status in myocardial infarction and renal complications. Singap Med J. 2007;48:137–42.
Ebbeling CB, Ludwig DS. Treating obesity in youth: should dietary glycemic load be a consideration? Adv Pediatr. 2001;48:179–212.
Edwin E, Sheeja E, Gupta VB, Jain DC. Fight diabetes the herbal way review. Express Pharma. 2008;1:41–2.
Oyedemi SO, Bradley G, Afolayan AJ. Ethnobotanical survey of medicinal plants used for the management of diabetes mellitus in the Nkonkobe Municipality of South Africa. J Med Plant Res. 2009;12:1040–4.
Kareru PG, Kenji GM, Gachanja AN, Keriko JM, Mungai G. Traditional medicines among the Embu and Mbeere peoples of Kenya. Afr J Trad Compl Alter Med. 2007;4:75–86.
Angenot L, Tits M. Isolation of a New Alkaloid (O-Acetylretuline) and a Triterpenoid (Friedelin) from Strychnos henningsii of Zaire. Planta Med. 1981;41:240–3.
Oyedemi SO, Bradley G, Afolayan AJ. Beneficial effect of aqueous stem bark extracts of Strychnos henningsii Gilg in streptozotocin-nicotinamide induced type 2 diabetic wistar rats. Int J Pharmacol. 2011;7:773–81.
van de Venter M, Roux S, Bungu LC, Louw J, Crouch NC, Grace OM, et al. Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. J Ethnopharmacol. 2008;119:81–6.
Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, Hasegawa J, et al. Screening system for the Maillard reaction inhibitor from natural products extracts. J Health Sci. 2002;48:520–6.
Sancheti S, Sancheti S, Seo SY. Evaluation of antiglycosidasae and anticholinesterase activities of Boehmerianivea. Pak J Pharm Sci. 2010;23:236–40.
Zhizhuang X, Storms R, Tsang A. Microplate-based carboxymethyl-cellulose assay for endoglucanase activity. Anal Biochem. 2005;342:176–8.
Lister E, Wilson P. Measurement of total phenolics and ABTS assay for antioxidant activity (personal communication). Lincoln: Crop Research Institute; 2001.
Benzie FF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–23.
Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT 4 vesicle trafficking. J Biol Chem. 2000;274:2593–6.
Rang HP, Dale MM, Ritters JM. The endocrine pancreas and the control of blood glucose. In: Barbara S, Beasley S, editors. Pharmacology. U. K. Longman group Ltd.; 1991. p. 403–10.
Lenzen S. The mechanisms of alloxan and streptozotocin induced diabetes. Diabetologia. 2008;51:216–26.
Greven WL, Smith JM, Rommes JH, Spronk PE. Accumulation of advanced glycation end (AGEs) products in intensive care patients: an observational prospective study. BMC Clin Pathol. 2010;10:4–9.
Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky L, Darnell J. Molecular cell biology. 5th ed. Freeman books; 2004.
Chen J, Sadowski HB, Kohanski RA, Wang L. Stat5 is a physiological substrate of the insulin receptor. Nat Acad Sci Proc. 1996;94:2295–300.
Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Nat Acad Sci Proc. 1997;94:9660–4.
Zierath JR, Wallberg-Henriksson H. From receptor to effector: insulin signal transduction in skeletal muscle from type II diabetic patients. Ann N Y Acad Sci. 2002;967:120–34.
Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–67.
Dominguez LJ, Davidoff AJ, Srinivas PR, Standley PR, Walsh MF, Sowers JR. Effects of metformin on tyrosine kinase activity, glucose transport, and intracellular calcium in rat vascular smooth muscle. Endocrinol. 1996;137:113–21.
Ines P, Jianjun B, Liyan P, Michael NS. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31:25–51.
Philippe G, Angenot L, Tits M, Frédérich M. About the toxicity of some Strychnos species and their alkaloids. Toxicon. 2004;44:405–16.
Philippe G, Prost E, Nuzillard JM, Zèches-Hanrot M, Tits M, Angenot L, et al. Strychnohexamine from Strychnos icaja, a naturally occuring trimeric indolomonoterpenic alkaloid. Tetrahedron. 2002;43:3387–90.
Neuwinger HD. Alkaloids in arrow poisons. In: Roberts MC, Wink M, editors. Alkaloids: biochemistry, ecology and medicinal applications. New York: Plenum Press; 1998. p. 45–84.
Ulrich P, Cerami A. Protein glycation, diabetes and aging. Recent Prog Horm Res. 2001;56:1–21.
Singh SN, Vats P, Suri S, Shyam R, Kumria MML, Ranganathan S. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001;76:269–77.
Kiho T, Yamane A, Hui J, Usui S, Ukai S. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Phytother Res. 2000;14:647–9.
Ani V, Akhilender NK. Antihyperglycaemic effect of polyphenolic components of black/bitter cumin seeds Centratherum anthelminticum (Willd.) Kuntz. Eur Food Res Technol. 2008;226:897–903.
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley P, Pridham JB. The relative activities of plant-derived polyphenolic flavonoid. Free Radic Res. 1995;22:375–83.
Acknowledgments
The authors thank National Research Foundation (NRF) of South Africa for their financial support.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oyedemi, S., Koekemoer, T., Bradley, G. et al. In vitro anti-hyperglycemia properties of the aqueous stem bark extract from Strychnos henningsii (Gilg). Int J Diabetes Dev Ctries 33, 120–127 (2013). https://doi.org/10.1007/s13410-013-0120-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-013-0120-8