Abstract
A sensor based on gold nanoparticle/double-walled carbon nanotube-modified glassy carbon electrode is prepared. Electrochemical behavior of dopamine hydrochloride at gold nanoparticle/double-walled carbon nanotube-modified glassy carbon electrode is investigated. A simple, sensitive, and inexpensive method for determination of dopamine hydrochloride is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNTs) have attracted much attention since their discovery; they are used in many fields because of their exceptional mechanical, electronic, and chemical properties. The advantages of CNTs for these applications could be increased electron transfer kinetics and apparent electrocatalytic effect for many reaction, CNTs have been employed for various electrochemical applications [1–4].
Gold nanoparticles (GNs) are particularly attractive for numerous investigations by virtue of their facile synthesis, the large specific surface area, high surface free energy, good conductivity, optical properties, high chemical ability, favorable biocompatibility, and catalytic applications [5–9].
Dopamine is the immediate precursor of epinephrine in the body. Exogenously administered, it produces direct stimulation of beta-1 receptors and variable stimulation of alpha receptors (peripheral vasoconstriction). It causes a release of norepinephrine from its storage sites. These actions result in increased myocardial contraction and increased renal blood flow and sodium excretion [10]. Dopamine hydrochloride (DPH) injection solution as a drug is a clear, practically colorless, sterile, pyrogen-free, aqueous solution for intravenous infusion after dilution. DPH has been used for treating all kinds of shock syndromes. It is very important to find a simple and sensitive method to determine content of DPH in clinical medicine. High performance liquid chromatography (HPLC) method [11] was described for the determination of DPH with expensive apparatus. Additionally, Wang et al. [12] reported the determination of DPH by fluorophotometry with narrow linear range, lower upper limit, and higher standard deviation. The spectrophotometry [13, 14] was also used for determination of DPH in injection solution and produce experimental error because of oxidation treatment for chromogenic reagent.
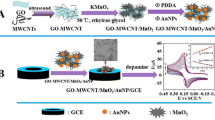
In the present work, a simple, sensitive, and inexpensive sensor based on GN/double-walled carbon nanotube (DWCNT)-modified glassy carbon electrode (GCE) is prepared. Electrochemical behavior of DPH at GN/DWCNT-modified GCE is investigated.
Experimental
DWCNTs were purchased from Shenzhen Nano-Technologies Port Co. Ltd. (China).
All other reagents were analytical grade. Double-distilled water was used throughout. 0.1 M phosphate buffer solution was prepared by dissolving 0.1 mol NaCl and 0.1 mol Na2HPO4 in the double-distilled water of 1,000 mL and adjusted desired pH values with 6 mol L−1HCl or 1 mol L−1NaOH aqueous solution.
For all electrochemical experiments, a CHI660B Electrochemical Analyzer (CHI, USA) was employed. The electrochemical cells consisted of a three electrode; a GN/DWCNT-modified GCE was used as working electrode. A platinum wire served as the counter electrode and a saturated calomel electrode was used as the reference electrode. The GN/DWCNT composite was characterized by transmission electron microscopy (TEM; JEM 2100, JEOL, Japan).
The DWCNTs were purified in boiling concentrated nitric acid for 3 h, followed by rinsing with double-distilled water and drying under atmosphere. Open-end DWCNTs with hydrophilic surface were thus obtained. Before modification, the GCE was polished with 0.05 μm alumina slurry on a polishing cloth, rinsed thoroughly with double-distilled water, and then sonicated in ethanol and double-distilled water for 10 min sequentially. The modifier suspension was prepared by dispersing 5.0 mg DWCNTs in the solvent of 5 mL N,N-dimethylformamide under sonication for 30 min. The DWCNT-modified GCE was prepared by casting 5 μL of the mentioned above black suspension on the GCE surface using a micropipette and left to dry at room temperature for 24 h, then the GNs were deposited at a voltage of −0.2 V for 30 s on the DWCNT/GCE that was immersed in 2 mg mL−1 HAuCl4 solution [15], and then washed in double-distilled water. The obtained GN/DWCNT-modified GCE was washed in double-distilled water. Before the voltammetric measurements, the modified electrode was cycled between −0.6 and 0.6 V (scan rate 100 mV s−1) in 0.1 M phosphate buffer solution of pH 7.3 for several times until acquiring the reproducible responses.
Results and discussion
SEM is applied to confirm the formation of a layer of the film on the GCE surface. The TEM image of GN/DWCNT is shown in Fig. 1, many GN/DWCNTs on the surface of GCE were observed. The phenomena indicated that GN/DWCNT had been assembled on the GCE surface.
The cyclic voltammery (CV) of modified GCE in the K3Fe(CN)6–K4Fe(CN)6 system is shown in Fig. 2. Compared with the bare GCE, the DWCNT/GCE and GN/DWCNT/GCE show higher peak current. The increase in voltammetric response of ferrocyanide is simply due to the intrinsic properties of the DWCNT/GCE and GN/DWCNT/GCE, which works to produce a larger peak current than that of the bare GCE. The real active surface area will be estimated. In a reversible process, the following Randles–Sevcik formula [16] has been used:
where, i p,a refers to the anodic peak current, n is the electron transfer number, A is the microscopic surface area of the electrode, D o is the diffusion coefficient of K3Fe(CN)6, C o is the bulk concentration of K3Fe(CN)6, and ν is the scan rate. From the slope of the plot of oxidation current (i p,a ) versus υ1/2, the microscopic areas can be calculated. The electrode surface area of the GN/DWCNT/GCE, DWCNT/GCE, and the bare GCE is 0.151, 0.104, and 0.074 cm2, respectively, indicating that the microscopic area of the GN/DWCNT-modified GCE increased significantly and was about 2.04 times larger than the microscopic area of the bare GCE. The anodic–cathodic peak separations are approximately 171, 137, and 176 mV for the GN/DWCNT/GCE, DWCNT/GCE, and bare GCE, respectively, indicating that this is a quasi-reversible process. To the best of our knowledge, a reversible process is controlled by the diffusion process; the diffusion process is also controlled by not only the scan rate but also the electrolyte in the solution and the functional groups such as -COOH, -OH, and Au+ etc. on the surface of the electrode.
The oxidation peaks for DPH at bare GCE, DWCNT/GCE, and GN/DWCNT/GCE are observed at 0.447, 0.233, and 0.252 V (Fig. 3), respectively, while the reduction peak for DPH at bare GCE, DWCNT/GCE, and GN/DWCNT/GCE are found at 0.068, 0.168, and 0.146 V, respectively. The oxidation potential for DPH at DWCNT/GCE and GN/DWCNT/GCE is less than that of DPH at bare GCE, the reduction potential for DPH at DWCNT/GCE and GN/DWCNT/GCE is more than that of DPH at bare GCE, and the peak current of DPH at GN/DWCNT/GCE is more than that of DPH at the DWCNT/GCE and bare GCE. The electrochemical response of GN/DWCNT/GCE in 0.1 M phosphate buffer solution of pH 7.3 is shown in Fig. 3 (inset). It can be seen that no peaks of blank CV on the bare GCE, DWCNT/GCE, and GN/DWCNT/GCE are found. These results indicated that the DWCNT- and GN/DWCNT-modified electrode promoted the electrochemical oxidation/reduction of DPH by considerably accelerating the rate of electron transfer. The functional groups such as -COOH, -OH, and Au+ on the surface of the DWCNTs may catalyze the redox of catechol in DPH. The current of DPH at DWCNT/GCE increases remarkably with the amount of DWCNTs on the surface of electrode increase; meanwhile, the background current of electrode increases with the amount of DWCNTs increase. However, the higher background current lead to narrow linear range and the sensitivity decreases with the amount of DWCNTs on the surface of GCE decrease. Thus, we select GN/DWCNT/GCE as the work electrode.
The information involving electrochemical mechanism usually can be obtained from the investigation of CVs in the different potential sweep rates. Therefore, the CV investigations for 50.0 mg L−1 DPH were performed on the surface of the GN/DWCNT-modified GCE in buffered solution of pH 7.3 at different potential sweep rates. Figure 4 illustrates the influence of scan rate on the CVs of 50.0 mg L−1 DPH in the range of 10–650 mV s−1. The linear relation between peak current (oxidation current, I pa; reduction current, I pc) of 50.0 mg L−1 DPH and square root of scan speed (ν1/2) in the range of 10–150 mV s−1 (Fig. 4, inset) indicates that a diffusion controls process on the surface of the modified electrode. The regression equations for this relationship are obtained as I pa = −327.84ν1/2−8.5800 (r = 0.996) and I pc = 344.318ν1/2−37.502, r = 0.996 (I, μA; ν, V s−1).
CVs of 50.0 mg L−1 DPH at GN/DWCNT/GCE with different scan rates. Insert plot of the peak current against the square root of scan rates. Other conditions are shown in Fig. 3
The relationship between the oxidation peak current and the concentration of DPH was examined by CVs on the surface of GN/DWCNT-modified GCE, and the results are shown in Fig. 5. Under the previously mentioned optimum conditions, the oxidation peak currents are proportional to DPH concentrations in the range of 2.00–25.00 mg L−1 in 0.1 M phosphate buffer solution of pH 7.3. The linear regression equation is obtained as c (mg L−1) = 0.2577 I pa (μA) − 3.6662 (R = 0.997). The detection limit (3σ/slope, where σ is the standard deviation of the intercept and s is the slope of the calibration curve) observed for DPH is 0.20 mg L−1.
CVs of different concentration DPH. Insert plot of the peak current against the concentration. Other conditions are as in Fig. 3
The repeatability of the modified electrode was investigated by repetitive recording at a fixed DPH concentration of 50.0 mg L−1. The relative standard deviation (RSD) for the peak currents in CVs based on six replicates was 1.4%, indicating excellent repeatability of the response of the modified electrode. Also, on using the GN/DWCNT-modified GCE daily and storing under ambient conditions over a period of 2 weeks, the electrode retained 97.8% of its initial peak current response for a DPH concentration of 50.0 mg L−1, which shows long-term stability of the film modifier on the surface of GCE. The results indicate that the modified electrode has an excellent repeatability and reproducibility.
The influence of some organic compounds and mineral salts in blood was tested. If the presence of an interferent altered the average current signal of 50.0 mg L–1 DPH concentration by less than ±5%, we considered that caused no interference. The results shows a 100-fold of glucose, K+, Ca2+, Zn2+, Mg2+, SO 2−4 , fivefold of ascorbic acid, uric acid, and large number of Na+, Cl−, H2PO −4 did not interfere with the determination, while epinephrine interfered severely. This suggests that the modified electrode had certain resistance to some interference.
To assess the applicability of the proposed method, the GN/DWCNT-modified GCE was used to determination of the content of DPH injection solution by applying CV method. 1–5 μL of 10.0 g L−1 DPH injection solution was diluted to 5.0 ml with 0.1 M phosphate buffer solution of pH 7.3, the determination results using the standard addition method are shown in Table 1. The recoveries are in the range from 96.7% to 109.0%, the content for the diluted DPH is obtained to be 2.01–10.10 mg L−1 with RSD of 1.6–2.3% (n = 6).
In conclusion, it was demonstrated that modification of GCE with GN/DWCNT is a simple and effective method for obtaining highly sensitive electrodes for determination of DPH. The procedure enables preparation of highly stable and reproducible uniform modifier films, which leads to a considerable enhancement in repeatability and reproducibility in the voltammetric measurements. High sensitivity and improved detection limit of the GN/DWCNT-modified GCE are promising for the determination of DPH injection solution.
References
Kruusenberg I, Matisen L, Jiang H, Huuppola M, Kontturi K, Tammeveski K (2010) Electrochemical reduction of oxygen on double-walled carbon nanotube modified glassy carbon electrodes in acid and alkaline solutions. Electrochem Commun 12:920–923
Scott CL, Pumera M (2011) Electroanalytical parameters of carbon nanotubes are inferior with respect to well defined surfaces of glassy carbon and EPPG. Electrochem Commun 13:213–216
Henstridge MC, Dickinson EJF, Aslanoglu M, Batchelor-McAuley C, Compton RG (2010) Voltammetric selectivity conferred by the modification of electrodes using conductive porous layers or films: the oxidation of dopamine on glassy carbon electrodes modified with multiwalled carbon nanotubes. Sensor Actuat B Chem 145:417–427
Nie J-Q, Zhang Q, Zhao M-Q, Huang J-Q, Wen Q, Cui Y, Qian W-Z, Wei F (2011) Synthesis of high quality single-walled carbon nanotubes on natural sepiolite and their use for phenol absorption. Carbon 49:1568–1580
Zhao W, Xu J, Shi C, Chen H (2006) Fabrication, characterization and application of gold nano-structured film. Electrochem Commun 8:773–778
Vidya R, Sreenivasan K (2010) Selective detection and estimation of C-reactive protein in serum using surface-functionalized gold nano-particles. Anal Chim Acta 662:186–192
Araki H, Fukuoka A, Sakamoto Y, Inagaki S, Sugimoto N, Fukushima Y, Ichikawa M (2003) Template synthesis and characterization of gold nano-wires and -particles in mesoporous channels of FSM-16. J Mol Catal A 199:95–102
Schleunitz A, Steffes H, Chabicovsky R, Obermeier E (2007) Optical gas sensitivity of a metaloxide multilayer system with gold-nano-clusters. Sensor Actuat B Chem 127:210–216
Qu S, Li H, Peng T, Gao Y, Qiu J, Zhu C (2004) Optical nonlinearities from transverse plasmon resonance in gold nano-rods. Mater Lett 58:1427–1430
Przedbroski S, Leviver M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM (1995) Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by instrastriatal injection of 6-hydroxydopamine. Neuroscience 67:631–647
Yang N, Li J, Zhang L (2003) Determination of dopamine hydrochloride in dopamine hydrochloride injection solution. Chin J Pharm Anal 23:232–233
Wang HY, Hui QS, Xu LX (2003) Fluorimetric determination of dopamine in pharmaceutical products and urine using ethylene diamine as the fluorigenic reagent. Anal Chim Acta 497:93–99
Nagaraja P, Vasantha RA, Sunitha KR (2001) A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 55:1039–1046
Lia Q, Li J, Yang Z (2007) Study of the sensitization of tetradecyl benzyl dimethyl ammonium chloride for spectrophotometric determination of dopamine hydrochloride using sodium 1,2-naphthoquinone-4-sulfonate as the chemical derivative chromogenic reagent. Anal Chim Acta 583:147–152
Michael OF, George DB, Mark TM (1999) Characterization of electrochemically deposited gold nanocrystals on glassy carbon electrodes. J Electroanal Chem 466:234–241
Kissinger PT, Heineman WR (1984) Laboratory techniques in electroanalytical chemistry. Marcel Dekker, NY, Chapter, 3
Acknowledgments
This research was supported by the National Science Foundation of China (Grant No. 20975043) and Jiangsu Higher Institution Key Basic Research Projects of Natural Science (grant no. 07KJA15012).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Song, Y.Z., Song, Y. & Zhong, H. Gold nanoparticle/double-walled carbon nanotube-modified glassy carbon electrode and its application. Gold Bull 44, 107–111 (2011). https://doi.org/10.1007/s13404-011-0016-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-011-0016-7