Abstract
Purpose
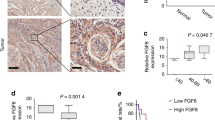
Metastatic lung squamous cell carcinoma (LUSC) is one of the most common causes of cancer death worldwide. As yet, however, the molecular mechanism underlying LUSC metastasis remains elusive. In this study, we report a novel mechanism involving signaling interactions between FGF19 and GLI2 that could drive the progression of LUSC.
Methods
The expression of FGF19 in human LUSC samples was assessed by immunohistochemistry. The concentration of FGF19 in serum samples was assessed by ELISA. RNA sequencing, scratch wound-healing, trans-well, GO analysis, GSEA, luciferase reporter, Western blotting, immunofluorescence and immunohistochemistry assays, as well as an animal model were used to investigate the molecular mechanism underlying FGF19 driven LUSC progression. The therapeutic effect of a GLI2 inhibitor was determined using both in vitro cellular and in vivo animal experiments.
Results
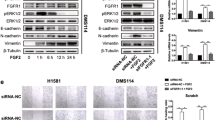
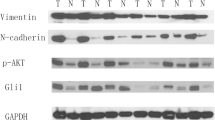
We found that FGF19, a member of the fibroblast growth factor family, plays a crucial role in the invasion and metastasis of LUSC, and identified GLI2 as an important downstream effector of FGF19 involved in metastasis. Surprisingly, we found that FGF19 and GLI2 could reciprocally induce the expression of each other, and form a positive feedback loop to promote LUSC cell invasion and metastasis. These findings were corroborated by an association between a poor prognosis of LUSC patients and FGF19/GLI2 co-expression. In addition, we found that the GLI inhibitor GANT61 could effectively reduce FGF19-mediated LUSC invasion and metastasis.
Conclusion
Our data suggest that FGF19 may serve as a novel biomarker for predicting metastatic LUSC. Intervening with the FGF19-GLI2 feedback loop may be a strategy for the treatment of FGF19-driven LUSC metastasis.






Similar content being viewed by others
Data availability
The RNA-seq data generated in this study are available in GEO under accession number GSE207588. Other data that support the findings of this study are available from the corresponding author upon request.
References
F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424 (2018)
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020)
C. Cheng, D. Liu, Z. Liu, M. Li, Y. Wang, B. Sun, R. Kong, H. Chen, G. Wang, L. Li, J. Hu, Y. Li, H. Chen, Z. Zhao, T. Zhang, S. Zhu, S. Pan, Positive feedback regulation of lncRNA TPT1-AS1 and ITGB3 promotes cell growth and metastasis in pancreatic cancer. Cancer Sci. 113(9), 2986–3001 (2022)
M. Ghomlaghi, A. Hart, N. Hoang, S. Shin, L.K. Nguyen, Feedback, crosstalk and competition: ingredients for Emergent Non-Linear Behaviour in the PI3K/mTOR Signalling Network. Int. J. Mol. Sci. 22(13), 6944 (2021)
S. Zhao, Y. Mi, B. Zheng, P. Wei, Y. Gu, Z. Zhang, Y. Xu, S. Cai, X. Li, D. Li, Highly-metastatic colorectal cancer cell released miR-181a-5p-rich extracellular vesicles promote liver metastasis by activating hepatic stellate cells and remodelling the tumour microenvironment. J. Extracell. Vesicles 11, e12186 (2022)
M. Katoh, M. Katoh, Evolutionary conservation of CCND1-ORAOV1-FGF19-FGF4 locus from zebrafish to human. Int. J. Mol. Med. 12, 45–50 (2003)
B. Angelin, T.E. Larsson, M. Rudling, Circulating fibroblast growth factors as metabolic regulators–a critical appraisal. Cell. Metab. 16, 693–705 (2012)
T. Inagaki, M. Choi, A. Moschetta, L. Peng, C.L. Cummins, J.G. McDonald, G. Luo, S.A. Jones, B. Goodwin, J.A. Richardson, R.D. Gerard, J.J. Repa, D.J. Mangelsdorf, S.A. Kliewer, Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell. Metab. 2, 217–225 (2005)
M. Choi, A. Moschetta, A.L. Bookout, L. Peng, M. Umetani, S.R. Holmstrom, K. Suino-Powell, H.E. Xu, J.A. Richardson, R.D. Gerard, D.J. Mangelsdorf, S.A. Kliewer, Identification of a hormonal basis for gallbladder filling. Nat. Med. 12, 1253–1255 (2006)
E. Somm, F.R. Jornayvaz, Fibroblast growth factor 15/19: from Basic Functions to therapeutic perspectives. Endocr. Rev. 39, 960–989 (2018)
H.I. Sussman, Apical or cervical? J. Am. Dent. Assoc. 118, 684 (1989)
X. Zhao, F. Xu, N.P. Dominguez, Y. Xiong, Z. Xiong, H. Peng, C. Shay, Y. Teng, FGFR4 provides the conduit to facilitate FGF19 signaling in breast cancer progression. Mol. Carcinog. 57, 1616–1625 (2018)
H. Nagamatsu, J. Teishima, K. Goto, H. Shikuma, H. Kitano, K. Shoji, S. Inoue, A. Matsubara, FGF19 promotes progression of prostate cancer. Prostate 75, 1092–1101 (2015)
D. Wang, J. Zhang, Z. Li, J. Han, Y. Gao, M. Chen, Y. Li, Upregulation of fibroblast growth factor 19 is Associated with the initiation of colorectal adenoma. Dig. Dis. 37, 214–225 (2019)
Y. Liu, M. Cao, Y. Cai, X. Li, C. Zhao, R. Cui, Dissecting the role of the FGF19-FGFR4 signaling pathway in Cancer Development and Progression. Front. Cell. Dev. Biol. 8, 95 (2020)
H.K. Ho, S. Pok, S. Streit, J.E. Ruhe, S. Hart, K.S. Lim, H.L. Loo, M.O. Aung, S.G. Lim, A. Ullrich, Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J. Hepatol. 50, 118–127 (2009)
F. Li, Z. Li, Q. Han, Y. Cheng, W. Ji, Y. Yang, S. Lu, W. Xia, Enhanced autocrine FGF19/FGFR4 signaling drives the progression of lung squamous cell carcinoma, which responds to mTOR inhibitor AZD2104. Oncogene 39, 3507–3521 (2020)
Y. Zhang, T. Wu, F. Li, Y. Cheng, Q. Han, X. Lu, S. Lu, W. Xia, FGF19 is Coamplified with CCND1 to promote proliferation in lung squamous cell carcinoma and their combined inhibition shows improved efficacy. Front. Oncol. 12, 846744 (2022)
X. Zhang, M. Kong, Z. Zhang, S. Xu, F. Yan, L. Wei, J. Zhou, FGF19 genetic amplification as a potential therapeutic target in lung squamous cell carcinomas. Thorac. Cancer 8, 655–665 (2017)
J. Chen, J. Shao, A. Shen, X. Zhu, X. Zhang, H. Sun, S. Wei, Y. Ling, Enhanced expression of FGF19 predicts poor prognosis in patients with non-small cell lung cancer. J. Thorac. Dis. 13, 1769–1784 (2021)
H. Zhao, F. Lv, G. Liang, X. Huang, G. Wu, W. Zhang, L. Yu, L. Shi, Y. Teng, FGF19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the GSK3beta/beta- catenin signaling cascade via FGFR4 activation. Oncotarget 7, 13575–13586 (2016)
L. Gao, X. Wang, Y. Tang, S. Huang, C.A. Hu, Y. Teng, FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J. Exp. Clin. Cancer Res. 36, 8 (2017)
L. Lang, Y. Xiong, N. Prieto-Dominguez, R. Loveless, C. Jensen, C. Shay, Y. Teng, FGF19/FGFR4 signaling axis confines and switches the role of melatonin in head and neck cancer metastasis. J. Exp. Clin. Cancer Res. 40, 93 (2021)
A.N. Sigafoos, B.D. Paradise, M.E. Fernandez-Zapico, Hedgehog/GLI signaling pathway: transduction, regulation, and implications for Disease. Cancers (Basel) 13(14), 3410 (2021)
E. Peer, S. Tesanovic, F. Aberger, Next-generation Hedgehog/GLI pathway inhibitors for Cancer Therapy. Cancers (Basel) 11(4), 538 (2019)
O. Nolan-Stevaux, J. Lau, M.L. Truitt, G.C. Chu, M. Hebrok, M.E. Fernandez-Zapico, D. Hanahan, GLI1 is regulated through smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 23, 24–36 (2009)
Z. Jagani, E.L. Mora-Blanco, C.G. Sansam, E.S. McKenna, B. Wilson, D. Chen, J. Klekota, P. Tamayo, P.T. Nguyen, M. Tolstorukov, P.J. Park, Y.J. Cho, K. Hsiao, S. Buonamici, S.L. Pomeroy, J.P. Mesirov, H. Ruffner, T. Bouwmeester, S.J. Luchansky, J. Murtie, J.F. Kelleher, M. Warmuth, W.R. Sellers, C.W. Roberts, M. Dorsch, Loss of the tumor suppressor Snf5 leads to aberrant activation of the hedgehog-gli pathway. Nat. Med. 16, 1429–1433 (2010)
Y. Wang, Q. Ding, C.J. Yen, W. Xia, J.G. Izzo, J.Y. Lang, C.W. Li, J.L. Hsu, S.A. Miller, X. Wang, D.F. Lee, J.M. Hsu, L. Huo, A.M. Labaff, D. Liu, T.H. Huang, C.C. Lai, F.J. Tsai, W.C. Chang, C.H. Chen, T.T. Wu, N.S. Buttar, K.K. Wang, Y. Wu, H. Wang, J. Ajani, M.C. Hung, The crosstalk of mTOR/S6K1 and hedgehog pathways. Cancer Cell. 21, 374–387 (2012)
W. Ji, Y. Yu, Z. Li, G. Wang, F. Li, W. Xia, S. Lu, FGFR1 promotes the stem cell-like phenotype of FGFR1-amplified non-small cell lung cancer cells through the hedgehog pathway. Oncotarget 7, 15118–15134 (2016)
L. Wang, H. Yang, Z. Lei, J. Zhao, Y. Chen, P. Chen, C. Li, Y. Zeng, Z. Liu, X. Liu, H.T. Zhang, Repression of TIF1gamma by SOX2 promotes TGF-beta-induced epithelial-mesenchymal transition in non-small-cell lung cancer. Oncogene 35, 867–877 (2016)
B. Gyorffy, P. Surowiak, J. Budczies, A. Lanczky, Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One 8, e82241 (2013)
P. Gupta, N. Gupta, N.M. Fofaria, A. Ranjan, S.K. Srivastava, HER2-mediated GLI2 stabilization promotes anoikis resistance and metastasis of breast cancer cells. Cancer Lett. 442, 68–81 (2019)
H. Nagao-Kitamoto, M. Nagata, S. Nagano, S. Kitamoto, Y. Ishidou, T. Yamamoto, S. Nakamura, A. Tsuru, M. Abematsu, Y. Fujimoto, M. Yokouchi, S. Kitajima, T. Yoshioka, S. Maeda, S. Yonezawa, S. Komiya, T. Setoguchi, GLI2 is a novel therapeutic target for metastasis of osteosarcoma. Int. J. Cancer 136, 1276–1284 (2015)
H. Zhang, Y. Wang, T. Chen, Y. Zhang, R. Xu, W. Wang, M. Cheng, Q. Chen, Aberrant activation of hedgehog signalling promotes cell migration and invasion via matrix metalloproteinase-7 in ovarian cancer cells. J. Cancer 10, 990–1003 (2019)
H. Saitsu, M. Komada, M. Suzuki, R. Nakayama, J. Motoyama, K. Shiota, M. Ishibashi, Expression of the mouse Fgf15 gene is directly initiated by sonic hedgehog signaling in the diencephalon and midbrain. Dev. Dyn. 232, 282–292 (2005)
Q. Tan, F. Li, G. Wang, W. Xia, Z. Li, X. Niu, W. Ji, H. Yuan, Q. Xu, Q. Luo, J. Zhang, S. Lu, Identification of FGF19 as a prognostic marker and potential driver gene of lung squamous cell carcinomas in chinese smoking patients. Oncotarget 7, 18394–18402 (2016)
D. Javelaud, V.I. Alexaki, S. Dennler, K.S. Mohammad, T.A. Guise, A. Mauviel, TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 71, 5606–5610 (2011)
Y. Katoh, M. Katoh, Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA (review). Int. J. Mol. Med. 22, 271–275 (2008)
X. Chen, S. Lingala, S. Khoobyari, J. Nolta, M.A. Zern, J. Wu, Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J. Hepatol. 55, 838–845 (2011)
B.N. Kholodenko, J.F. Hancock, W. Kolch, Signalling ballet in space and time. Nat. Rev. Mol. Cell. Biol. 11, 414–426 (2010)
W. Gu, H. Shen, L. Xie, X. Zhang, J. Yang, The role of feedback loops in targeted therapy for pancreatic cancer. Front. Oncol. 12, 800140 (2022)
E.J. Tolosa, M.G. Fernandez-Barrena, E. Iguchi, A.L. McCleary-Wheeler, R.M. Carr, L.L. Almada, L.F. Flores, R.E. Vera, G.W. Alfonse, D.L. Marks, T.L. Hogenson, A.M. Vrabel, I.P. Horn, A.N. Koenig, S.L. Safgren, A.N. Sigafoos, M. Erkan, P.A. Romecin-Duran, A. Sarabia Gonzalez, B. Zhou, D. Javelaud, V. Marsaud, R.P. Graham, A. Mauviel, S.F. Elsawa, M.E. Fernandez-Zapico, GLI1/GLI2 functional interplay is required to control Hedgehog/GLI targets gene expression. Biochem. J. 477, 3131–3145 (2020)
Y. Katoh, M. Katoh, Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr. Mol. Med. 9, 873–886 (2009)
X. Yang, W. Sun, X. Jing, Q. Zhang, H. Huang, Z. Xu, C/EBP homologous protein promotes Sonic hedgehog secretion from type II alveolar epithelial cells and activates hedgehog signaling pathway of fibroblast in pulmonary fibrosis. Respir Res. 23, 86 (2022)
A. Saito, S. Kanemoto, Y. Zhang, R. Asada, K. Hino, K. Imaizumi, Chondrocyte proliferation regulated by secreted luminal domain of ER stress transducer BBF2H7/CREB3L2. Mol. Cell. 53, 127–139 (2014)
Acknowledgements
The authors thank to all those who financed the study.
Funding
This work was supported by grants from the Science and Technology Commission of Shanghai Municipality (21ZR1433100), the State Key Laboratory of Oncogenes and Related Genes (KF2122) and SJTU funding (YG2022ZD016).
Author information
Authors and Affiliations
Contributions
Conception and design: Y.Z. and W.X.; Data acquisition and analysis: Y.Z., T.W., Y.W., Z.C., J.C. and W.X.; writing and original draft preparation: Y.Z., T.W. and W.X.; critical review and editing: Y.Z., T.W. and W.X.; studies related to clinical samples: Y.W., S.L.; supervision: W.X.; funding acquisition: W.X. All authors have seen and approved the final draft of the manuscript before submission. The work reported has been performed by the authors, unless clearly specified in the text.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Research Ethics Committee of the School of Biomedical Engineering, Shanghai Jiao Tong University (Shanghai, China). All patients were informed of the study and consented to the use of samples for research purposes. All animal experiments were performed following the regulations and internal biosafety and bioethics guidelines of the Research Ethics Committee of the School of Biomedical Engineering, Shanghai Jiao Tong University (Shanghai, China).
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Wu, T., Wang, Y. et al. Reciprocal FGF19-GLI2 signaling induces epithelial-to-mesenchymal transition to promote lung squamous cell carcinoma metastasis. Cell Oncol. 46, 437–450 (2023). https://doi.org/10.1007/s13402-022-00760-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-022-00760-y