Abstract
Purpose
Cervical cancer remains a major cause of cancer-related death in women, especially in developing countries. Previously, we found that the acetylation levels of chloride intracellular channel 1 (CLIC1) at lysine 131 were increased in cervical cancer tissues using a label-free proteomics approach. The aim of this study was to further determine the role of CLIC1 expression and its acetylation in cervical cancer.
Methods
CLIC1 expression and its implications for the prognosis of cervical cancer were analyzed using primary patient samples and cells, and the Gene Expression Profiling Interactive Analysis (GEPIA) database (gepia.cancer-pku.cn). The effect of CLIC1 on cervical cancer cells was evaluated using Cell Counting Kit (CCK)-8, flow cytometry, scratch wound healing, transwell, Western blotting and co-immunoprecipitation (Co-IP) assays. In vivo tumor growth was assessed using mouse xenograft models.
Results
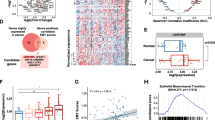
We found that CLIC1 expression was increased in cervical cancer tissues and cells and that patients with a high CLIC1 expression tended to have a shorter overall survival time. Knockdown of CLIC1 significantly reduced in vitro cervical cancer cell proliferation, migration and invasion, and in vivo tumorigenesis. At the molecular level, we found that nuclear factor kappa B (NF-κB) activity was positively regulated by CLIC1. Pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-κB, attenuated the tumor-promoting effect of CLIC1. Moreover, we found that CLIC1 acetylation at K131 was upregulated in cervical cancer cells, which stabilized CLIC1 by inhibiting its ubiquitynation. Substitution of K131 inhibited CLIC1 ubiquitynation and promoted in vitro cervical cancer cell proliferation, migration and invasion, and in vivo tumor growth. In addition, we found that acetyltransferase HAT1 was responsible for CLIC1 acetylation at K131.
Conclusion
Our data indicate that CLIC1 acts as a tumor promoter in cervical cancer, suggesting a potential treatment strategy for cervical cancer by regulating CLIC1 expression and/or acetylation.







Similar content being viewed by others
References
F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424 (2018)
P.A. Cohen, A. Jhingran, A. Oaknin, L. Denny, Cervical cancer. Lancet 393, 169–182 (2019)
R. Wei, G. Jiang, M. Lv, S. Tan, X. Wang, Y. Zhou, T. Cheng, X. Gao, X. Chen, W. Wang, C. Zou, F. Li, X. Ma, J. Hu, D. Ma, D. Luo, L. Xi, TMTP1-modified indocyanine green-loaded polymeric micelles for targeted imaging of cervical cancer and metastasis sentinel lymph node in vivo. Theranostics 9, 7325–7344 (2019)
S. Averaimo, R.H. Milton, M.R. Duchen, M. Mazzanti, Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett 584, 2076–2084 (2010)
P. Samaras, T. Schmidt, M. Frejno, S. Gessulat, M. Reinecke, A. Jarzab, J. Zecha, J. Mergner, P. Giansanti, H.C. Ehrlich, S. Aiche, J. Rank, H. Kienegger, H. Krcmar, B. Kuster, M. Wilhelm, ProteomicsDB: A multi-omics and multi-organism resource for life science research. Nucleic Acids Res 48, D1153–D1163 (2020)
J.Y. Yang, J.Y. Jung, S.W. Cho, H.J. Choi, S.W. Kim, S.Y. Kim, H.J. Kim, C.H. Jang, M.G. Lee, J. Han, C.S. Shin, Chloride intracellular channel 1 regulates osteoblast differentiation. Bone 45, 1175–1185 (2009)
G. Novarino, C. Fabrizi, R. Tonini, M.A. Denti, F. Malchiodi-Albedi, G.M. Lauro, B. Sacchetti, S. Paradisi, A. Ferroni, P.M. Curmi, S.N. Breit, M. Mazzanti, Involvement of the intracellular ion channel CLIC1 in microglia-mediated beta-amyloid-induced neurotoxicity. J Neurosci 24, 5322–5330 (2004)
S. Averaimo, M. Gritti, E. Barini, L. Gasparini, M. Mazzanti, CLIC1 functional expression is required for cAMP-induced neurite elongation in post-natal mouse retinal ganglion cells. J Neurochem 131, 444–456 (2014)
X. Jiang, Y. Liu, G. Wang, Y. Yao, C. Mei, X. Wu, W. Ma, Y. Yuan, Up-regulation of CLIC1 activates MYC signaling and forms a positive feedback regulatory loop with MYC in hepatocellular carcinoma. Am J Cancer Res 10, 2355–2370 (2020)
Q. Ding, M. Li, X. Wu, L. Zhang, W. Wu, Q. Ding, H. Weng, X. Wang, Y. Liu, CLIC1 overexpression is associated with poor prognosis in gallbladder cancer. Tumour Biol 36, 193–198 (2015)
J. Lu, Q. Dong, B. Zhang, X. Wang, B. Ye, F. Zhang, X. Song, G. Gao, J. Mu, Z. Wang, F. Ma, J. Gu, Chloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancer. Med Oncol 32, 616 (2015)
M. Setti, N. Savalli, D. Osti, C. Richichi, M. Angelini, P. Brescia, L. Fornasari, M.S. Carro, M. Mazzanti, G. Pelicci, Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J Natl Cancer Inst 105, 1644–1655 (2013)
A. Nesiu, A.M. Cimpean, R.A. Ceausu, A. Adile, I. Ioiart, C. Porta, M. Mazzanti, T.C. Camerota, M. Raica, Intracellular chloride ion channel protein-1 expression in clear cell renal cell carcinoma. Cancer Genomics Proteomics 16, 299–307 (2019)
B. Singha, S.L. Harper, A.R. Goldman, B.G. Bitler, K.M. Aird, M.E. Borowsky, M.G. Cadungog, Q. Liu, R. Zhang, S. Jean, R. Drapkin, D.W. Speicher, CLIC1 and CLIC4 complement CA125 as a diagnostic biomarker panel for all subtypes of epithelial ovarian cancer. Sci Rep 8, 14725 (2018)
L. Kong, Q. Wu, L. Zhao, J. Ye, N. Li, H. Yang, Upregulated lncRNA-UCA1 contributes to metastasis of bile duct carcinoma through regulation of miR-122/CLIC1 and activation of the ERK/MAPK signaling pathway. Cell Cycle 18, 1212–1228 (2019)
H. Katayama, P. Tsou, M. Kobayashi, M. Capello, H. Wang, F. Esteva, M.L. Disis, S. Hanash, A plasma protein derived TGFbeta signature is a prognostic indicator in triple negative breast cancer. NPJ Precis Oncol 3, 10 (2019)
M.A. Francisco, S. Wanggou, J.J. Fan, W. Dong, X. Chen, A. Momin, N. Abeysundara, H.K. Min, J. Chan, R. McAdam, M. Sia, R.J. Pusong, S. Liu, N. Patel, V. Ramaswamy, N. Kijima, L.Y. Wang, Y. Song, R. Kafri, M.D. Taylor, X. Li, X. Huang, Chloride intracellular channel 1 cooperates with potassium channel EAG2 to promote medulloblastoma growth. J Exp Med 217, e20190971 (2020)
J. Feng, J. Xu, Y. Xu, J. Xiong, T. Xiao, C. Jiang, X. Li, Q. Wang, J. Li, Y. Li, CLIC1 promotes the progression of oral squamous cell carcinoma via integrins/ERK pathways. Am J Transl Res 11, 557–571 (2019)
X. Wei, J. Li, H. Xie, H. Wang, J. Wang, X. Zhang, R. Zhuang, D. Lu, Q. Ling, L. Zhou, X. Xu, S. Zheng, Chloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspin. J Gastroenterol Hepatol 30, 208–216 (2015)
E. Verdin, M. Ott, 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol 16, 258–264 (2015)
S.S. Vadvalkar, C.N. Baily, S. Matsuzaki, M. West, Y.A. Tesiram, K.M. Humphries, Metabolic inflexibility and protein lysine acetylation in heart mitochondria of a chronic model of type 1 diabetes. Biochem J 449, 253–261 (2013)
H. Kozuka-Hata, A. Kitamura, T. Hiroki, A. Aizawa, K. Tsumoto, J.I. Inoue, M. Oyama, System-wide analysis of protein acetylation and ubiquitination reveals a diversified regulation in human cancer cells. Biomolecules 10, 411 (2020)
S. Nagarajan, S.V. Rao, J. Sutton, D. Cheeseman, S. Dunn, E.K. Papachristou, J.G. Prada, D.L. Couturier, S. Kumar, K. Kishore, C.S.R. Chilamakuri, S.E. Glont, E. Archer Goode, C. Brodie, N. Guppy, R. Natrajan, A. Bruna, C. Caldas, A. Russell, R. Siersbaek, K. Yusa, I. Chernukhin, J.S. Carroll, ARID1A influences HDAC1/BRD4 activity, intrinsic proliferative capacity and breast cancer treatment response. Nat Genet 52, 187–197 (2020)
E.M. Da Costa, G. Armaos, G. McInnes, A. Beaudry, G. Moquin-Beaudry, V. Bertrand-Lehouillier, M. Caron, C. Richer, P. St-Onge, J.R. Johnson, N. Krogan, Y. Sai, M. Downey, M. Rafei, M. Boileau, K. Eppert, E. Flores-Diaz, A. Haman, T. Hoang, D. Sinnett, C. Beausejour, S. McGraw, N.J. Raynal, Heart failure drug proscillaridin a targets MYC overexpressing leukemia through global loss of lysine acetylation. J Exp Clin Cancer Res 38, 251 (2019)
C. Li, Y. Chen, H. Zhu, X. Zhang, L. Han, Z. Zhao, J. Wang, L. Ning, W. Zhou, C. Lu, L. Xu, J. Sang, Z. Feng, Y. Zhang, X. Lou, X. Bo, B. Zhu, C. Yu, M. Zheng, Y. Li, J. Sun, Z. Shen, Inhibition of histone deacetylation by MS-275 alleviates colitis by activating the vitamin D receptor. J Crohns Colitis 14, 1103–1118 (2020)
S.J. Marzi, S.K. Leung, T. Ribarska, E. Hannon, A.R. Smith, E. Pishva, J. Poschmann, K. Moore, C. Troakes, S. Al-Sarraj, S. Beck, S. Newman, K. Lunnon, L.C. Schalkwyk, J. Mill, A histone acetylome-wide association study of Alzheimer's disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat Neurosci 21, 1618–1627 (2018)
L. Zhang, W. Wang, S. Zhang, Y. Wang, W. Guo, Y. Liu, Y. Wang, Y. Zhang, Identification of lysine acetylome in cervical cancer by label-free quantitative proteomics. Cancer Cell Int 20, 182 (2020)
X. Yue, Y. Cui, Q. You, Y. Lu, J. Zhang, MicroRNA124 negatively regulates chloride intracellular channel 1 to suppress the migration and invasion of liver cancer cells. Oncol Rep 42, 1380–1390 (2019)
D.L. Zheng, Q.L. Huang, F. Zhou, Q.J. Huang, J.Y. Lin, X. Lin, PA28beta regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1. J Cell Biochem 113, 1537–1546 (2012)
R. Sen, D. Baltimore, Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46, 705–716 (1986)
S. Tilborghs, J. Corthouts, Y. Verhoeven, D. Arias, C. Rolfo, X.B. Trinh, P.A. van Dam, The role of nuclear factor-kappa B signaling in human cervical cancer. Crit Rev Oncol Hematol 120, 141–150 (2017)
J.D. Kearns, S. Basak, S.L. Werner, C.S. Huang, A. Hoffmann, IkappaBepsilon provides negative feedback to control NF-kappaB oscillations, signaling dynamics, and inflammatory gene expression. J Cell Biol 173, 659–664 (2006)
Y. Xia, S. Shen, I.M. Verma, NF-kappaB, an active player in human cancers. Cancer Immunol Res 2, 823–830 (2014)
R. Domingo-Fernandez, R.C. Coll, J. Kearney, S. Breit, L.A.J. O'Neill, The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1beta transcription and activate the NLRP3 inflammasome. J Biol Chem 292, 12077–12087 (2017)
C. Rebe, F. Ghiringhelli, Interleukin-1beta and cancer. Cancers (Basel) 12, 1791 (2020)
S. Kaypee, D. Sudarshan, M.K. Shanmugam, D. Mukherjee, G. Sethi, T.K. Kundu, Aberrant lysine acetylation in tumorigenesis: Implications in the development of therapeutics. Pharmacol Ther 162, 98–119 (2016)
R. Lin, R. Tao, X. Gao, T. Li, X. Zhou, K.L. Guan, Y. Xiong, Q.Y. Lei, Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol Cell 51, 506–518 (2013)
W. He, L. Wei, Q. Zou, Research progress in protein posttranslational modification site prediction. Brief Funct Genomics 18, 220–229 (2018)
S. Kleff, E.D. Andrulis, C.W. Anderson, R. Sternglanz, Identification of a gene encoding a yeast histone H4 acetyltransferase. J Biol Chem 270, 24674–24677 (1995)
M.R. Parthun, J. Widom, D.E. Gottschling, The major cytoplasmic histone acetyltransferase in yeast: Links to chromatin replication and histone metabolism. Cell 87, 85–94 (1996)
A.J. Sadler, B.A. Suliman, L. Yu, X. Yuan, D. Wang, A.T. Irving, S.T. Sarvestani, A. Banerjee, A.S. Mansell, J.P. Liu, S. Gerondakis, B.R. Williams, D. Xu, The acetyltransferase HAT1 moderates the NF-kappaB response by regulating the transcription factor PLZF. Nat Commun 6, 6795 (2015)
P.A. Agudelo Garcia, P. Nagarajan, M.R. Parthun, Hat1-dependent lysine acetylation targets diverse cellular functions. J Proteome Res 19, 1663–1673 (2020)
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81772274), the Natural Science Foundation of Heilongjiang Province (No. H2017045) and the Key Project of the Petrel Foundation of Harbin Medical University Cancer Hospital (No. JJZD2020-07). In addition, the study was supported by the Beijing Medical Award Foundation (No.YXJL-2020-0417-0043 and No.YXJL-2020-0510-0044) and the Heilongjiang University student innovation and Entrepreneurship training project (No.202011230002).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
WYX and CXW conceived and designed the study. WWY, LX and XY conducted the experiments. WWY, GWK, YH and ZL analyzed the data. WWY and LX wrote the manuscript. WYX and CXW modified the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Harbin Medical University and written informed consent was obtained from all patients. All animal experiments were performed in line with the Guide for the Care and Use of Laboratory Animal by the National Institutes of Health.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article title is present on Research Square as pre-print and can be accessed on https://www.researchsquare.com/article/rs-26424/v1.
Supplementary Information
ESM 1
(PNG 777 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Li, X., Xu, Y. et al. Acetylation-stabilized chloride intracellular channel 1 exerts a tumor-promoting effect on cervical cancer cells by activating NF-κB. Cell Oncol. 44, 557–568 (2021). https://doi.org/10.1007/s13402-020-00582-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00582-w