Abstract
Background
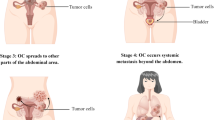
Ovarian cancer is the most lethal gynecologic cancer and the fifth leading cause of cancer-related mortality in women worldwide. Despite various attempts to improve the diagnosis and therapy of ovarian cancer patients, the survival rate for these patients is still dismal, mainly because most of them are diagnosed at a late stage. Up to 90% of ovarian cancers arise from neoplastic transformation of ovarian surface epithelial cells, and are usually referred to as epithelial ovarian cancer (EOC). Unlike most human cancers, which are disseminated through blood-borne metastatic routes, EOC has traditionally been thought to be disseminated through direct migration of ovarian tumor cells to the peritoneal cavity and omentum via peritoneal fluid. It has recently been shown, however, that EOC can also be disseminated through blood-borne metastatic routes, challenging previous thoughts about ovarian cancer metastasis.
Conclusions
Here, we review our current understanding of the most updated cellular and molecular mechanisms underlying EOC metastasis and discuss in more detail two main metastatic routes of EOC, i.e., transcoelomic metastasis and hematogenous metastasis. The emerging concept of blood-borne EOC metastasis has led to exploration of the significance of circulating tumor cells (CTCs) as novel and non-invasive prognostic markers in this daunting cancer. We also evaluate the role of tumor stroma, including cancer associated fibroblasts (CAFs), tumor associated macrophages (TAMs), endothelial cells, adipocytes, dendritic cells and extracellular matrix (ECM) components in EOC growth and metastasis. Lastly, we discuss therapeutic approaches for targeting EOC. Unraveling the mechanisms underlying EOC metastasis will open up avenues to the design of new therapeutic options. For instance, understanding the molecular mechanisms involved in the hematogenous metastasis of EOC, the biology of CTCs, and the detailed mechanisms through which EOC cells take advantage of stromal cells may help to find new opportunities for targeting EOC metastasis.


Similar content being viewed by others
References
R.L. Siegel, K.D. Miller, A. Jemal, Cancer Statistics, 2017. CA Cancer J. Clin. 67, 7–30 (2017)
Cancer Stat Facts: Ovarian Cancer 2019 [June 2019]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html
L.H. Smith, C.R. Morris, S. Yasmeen, A. Parikh-Patel, R.D. Cress, P.S. Romano, Ovarian cancer: can we make the clinical diagnosis earlier? Cancer 104, 1398–1407 (2005)
T.L. Yeung, C.S. Leung, K.P. Yip, C.L.A. Yeung, S.T. Wong, S.C. Mok, Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: cell and molecular processes in cancer metastasis. Am. J. Phys. Cell Phys. 309, C444–C456 (2015)
N. Auersperg, A.S. Wong, K.C. Choi, S.K. Kang, P.C. Leung, Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr. Rev. 22, 255–288 (2001)
C.L. Chaffer, R.A. Weinberg, A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011)
M. Yousefi, T. Bahrami, A. Salmaninejad, R. Nosrati, P. Ghaffari, S.H. Ghaffari, Lung cancer-associated brain metastasis: Molecular mechanisms and therapeutic options. Cell. Oncol. 40, 419–441 (2017)
K.R. Hess, G.R. Varadhachary, S.H. Taylor, W. Wei, M.N. Raber, R. Lenzi, J.L. Abbruzzese, Metastatic patterns in adenocarcinoma. Cancer 106, 1624–1633 (2006)
J. Budczies, M. von Winterfeld, F. Klauschen, M. Bockmayr, J.K. Lennerz, C. Denkert, T. Wolf, A. Warth, M. Dietel, I. Anagnostopoulos, W. Weichert, D. Wittschieber, A. Stenzinger, The landscape of metastatic progression patterns across major human cancers. Oncotarget 6, 570–583 (2015)
A.C. Obenauf, J. Massague, Surviving at a distance: organ specific metastasis. Trends Cancer 1, 76–91 (2015)
O. Akin, E. Sala, C.S. Moskowitz, N. Ishill, R.A. Soslow, D.S. Chi, H. Hricak, Perihepatic metastases from ovarian cancer: sensitivity and specificity of CT for the detection of metastases with and those without liver parenchymal invasion. Radiology 248, 511–517 (2008)
I.J. Fidler, G. Poste, The “seed and soil” hypothesis revisited. Lancet Oncol. 9, 808 (2008)
R.R. Langley, I.J. Fidler, The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128, 2527–2535 (2011)
I.J. Fidler, The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003)
N. Ribelles, A. Santonja, B. Pajares, C. Llácer, E. Alba, The seed and soil hypothesis revisited: Current state of knowledge of inherited genes on prognosis in breast cancer. Cancer Treat. Rev. 40, 293–299 (2014)
S. Pradeep, S.W. Kim, S.Y. Wu, M. Nishimura, P. Chaluvally-Raghavan, T. Miyake, C.V. Pecot, S.J. Kim, H.J. Choi, F.Z. Bischoff, J.A. Mayer, L. Huang, A.M. Nick, C.S. Hall, C. Rodriguez-Aguayo, B. Zand, H.J. Dalton, T. Arumugam, H.J. Lee, H.D. Han, M.S. Cho, R. Rupaimoole, L.S. Mangala, V. Sehgal, S.C. Oh, J. Liu, J.S. Lee, R.L. Coleman, P. Ram, G. Lopez-Berestein, I.J. Fidler, A.K. Sood, Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell 26, 77–91 (2014)
K. Hibbs, K.M. Skubitz, S.E. Pambuccian, R.C. Casey, K.M. Burleson, T.R. Oegema, J.J. Thiele, S.M. Grindle, R.L. Bliss, A.P.N. Skubitz, Differential Gene Expression in Ovarian Carcinoma : Identification of Potential Biomarkers. Am. J. Pathol. 165, 397–414 (2004)
J. Bayani, J.D. Brenton, P.F. Macgregor, B. Beheshti, M. Albert, D. Nallainathan, J. Karaskova, B. Rosen, J. Murphy, S. Laframboise, B. Zanke, J.A. Squire, Parallel analysis of sporadic primary ovarian carcinomas by spectral karyotyping, comparative genomic hybridization, and expression microarrays. Cancer Res. 62, 3466–3476 (2002)
A. Fishman, E. Shalom-Paz, M. Fejgin, E. Gaber, M. Altaras, A. Amiel, Comparing the genetic changes detected in the primary and secondary tumor sites of ovarian cancer using comparative genomic hybridization. Int. J. Gynecol. Cancer 15, 261–266 (2005)
D. Caserta, M. Benkhalifa, M. Baldi, F. Fiorentino, M. Qumsiyeh, M. Moscarini, Genome profiling of ovarian adenocarcinomas using pangenomic BACs microarray comparative genomic hybridization. Mol. Cytogenet. 1, 10 (2008)
D.S. Tan, R. Agarwal, S.B. Kaye, Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 7, 925–934 (2006)
E. Lengyel, Ovarian cancer development and metastasis. Am. J. Pathol. 177, 1053–1064 (2010)
D. Tarin, J.E. Price, M.G. Kettlewell, R.G. Souter, A.C. Vass, B. Crossley, Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res. 44, 3584–3592 (1984)
K.M. Nieman, H.A. Kenny, C.V. Penicka, A. Ladanyi, R. Buell-Gutbrod, M.R. Zillhardt, I.L. Romero, M.S. Carey, G.B. Mills, G.S. Hotamisligil, S.D. Yamada, M.E. Peter, K. Gwin, E. Lengyel, Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498 (2011)
T.R. Adib, S. Henderson, C. Perrett, D. Hewitt, D. Bourmpoulia, J. Ledermann, C. Boshoff, Predicting biomarkers for ovarian cancer using gene-expression microarrays. Br. J. Cancer 90, 686–692 (2004)
J. Bayani, J.D. Brenton, P.F. Macgregor, B. Beheshti, M. Albert, D. Nallainathan, J. Karaskova, B. Rosen, J. Murphy, S. Laframboise, B. Zanke, J.A. Squire, Parallel analysis of sporadic primary ovarian carcinomas by spectral karyotyping, comparative genomic hybridization, and expression microarrays. Cancer Res. 62, 3466–3476 (2002)
O. Israeli, W.H. Gotlieb, E. Friedman, J. Korach, E. Friedman, B. Goldman, A. Zeltser, G. Ben-Baruch, S. Rienstein, A. Aviram-Goldring, Genomic analyses of primary and metastatic serous epithelial ovarian cancer. Cancer Genet. Cytogenet. 154, 16–21 (2004)
A. Fishman, E. Shalom-Paz, M. Fejgin, E. Gaber, M. Altaras, A. Amiel, Comparing the genetic changes detected in the primary and secondary tumor sites of ovarian cancer using comparative genomic hybridization. Int. J. Gynecol. Cancer 15, 261–266 (2005)
F. van Roy, G. Berx, The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 65, 3756–3788 (2008)
M. Rosso, B. Majem, L. Devis, L. Lapyckyj, M.J. Besso, M. Llaurado, M.F. Abascal, M.L. Matos, L. Lanau, J. Castellvi, J.L. Sanchez, A. Perez Benavente, A. Gil-Moreno, J. Reventos, A. Santamaria Margalef, M. Rigau, M.H. Vazquez-Levin, E-cadherin: A determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS One 12, e0184439 (2017)
T. Imai, A. Horiuchi, C. Wang, K. Oka, S. Ohira, T. Nikaido, I. Konishi, Hypoxia attenuates the expression of E-Cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am. J. Pathol. 163, 1437–1447 (2003)
C. Faleiro-Rodrigues, I. Macedo-Pinto, D. Pereira, V.M. Ferreira, C.S. Lopes, Association of E-cadherin and beta-catenin immunoexpression with clinicopathologic features in primary ovarian carcinomas. Hum. Pathol. 35, 663–669 (2004)
S. Heerboth, G. Housman, M. Leary, M. Longacre, S. Byler, K. Lapinska, A. Willbanks, S. Sarkar, EMT and tumor metastasis. Clin. Transl. Med. 4, 6 (2015)
S. Zhang, C. Balch, M.W. Chan, H.C. Lai, D. Matei, J.M. Schilder, P.S. Yan, T.H. Huang, K.P. Nephew, Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 68, 4311–4320 (2008)
D. Vergara, B. Merlot, J.P. Lucot, P. Collinet, D. Vinatier, I. Fournier, M. Salzet, Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 291, 59–66 (2010)
A.N. Corps, H.M. Sowter, S.K. Smith, Hepatocyte growth factor stimulates motility, chemotaxis and mitogenesis in ovarian carcinoma cells expressing high levels of c-MET. Int. J. Cancer 73, 151–155 (1997)
M. Korpal, Y. Kang, The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 5, 115–119 (2008)
C. Wu, J. Cipollone, S. Maines-Bandiera, C. Tan, A. Karsan, N. Auersperg, C.D. Roskelley, The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation 76, 193–205 (2008)
L. Seguin, J.S. Desgrosellier, S.M. Weis, D.A. Cheresh, Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 25, 234–240 (2015)
N. Ahmed, F. Pansino, R. Clyde, P. Murthi, M.A. Quinn, G.E. Rice, M.V. Agrez, S. Mok, M.S. Baker, Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis 23, 237–244 (2002)
R.P. Czekay, D.J. Loskutoff, Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp. Biol. Med. (Maywood) 229, 1090–1096 (2004)
C.P. Carmignani, T.A. Sugarbaker, C.M. Bromley, P.H. Sugarbaker, Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 22, 465–472 (2003)
L. Xu, J. Yoneda, C. Herrera, J. Wood, J.J. Killion, I.J. Fidler, Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int. J. Oncol. 16, 445–454 (2000)
D. Belotti, P. Paganoni, L. Manenti, A. Garofalo, S. Marchini, G. Taraboletti, R. Giavazzi, Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 63, 5224–5229 (2003)
R.C. Casey, A.P. Skubitz, CD44 and beta1 integrins mediate ovarian carcinoma cell migration toward extracellular matrix proteins. Clin. Exp. Metastasis 18, 67–75 (2000)
F. Balkwill, Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 (2004)
K. Gawrychowski, G. Szewczyk, E. Skopińska-Różewska, M. Małecki, E. Barcz, P. Kamiński, M. Miedzińska-Maciejewska, W. Śmiertka, D. Szukiewicz, P. Skopiński, the angiogenic activity of ascites in the course of ovarian cancer as a marker of disease progression. Dis. Markers 2014, 683757 (2014)
N. Ahmed, K.L. Stenvers, Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front. Oncol. 3, 256 (2013)
J.C. Pease, M. Brewer, J.S. Tirnauer, Spontaneous spheroid budding from monolayers: a potential contribution to ovarian cancer dissemination. Biol. Open 1, 622–628 (2012)
K.M. Burleson, R.C. Casey, K.M. Skubitz, S.E. Pambuccian, T.R. Oegema Jr., A.P. Skubitz, Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol. Oncol. 93, 170–181 (2004)
M. Wintzell, E. Hjerpe, E. Avall Lundqvist, M. Shoshan, Protein markers of cancer-associated fibroblasts and tumor-initiating cells reveal subpopulations in freshly isolated ovarian cancer ascites. BMC Cancer 12, 359 (2012)
B. Davidson, C.G. Trope, R. Reich, The role of the tumor stroma in ovarian cancer. Front. Oncol. 4, 104 (2014)
E.K. Colvin, Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front. Oncol. 4, 137 (2014)
V.M. Abrahams, S.L. Straszewski, M. Kamsteeg, B. Hanczaruk, P.E. Schwartz, T.J. Rutherford, G. Mor, Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 63, 5573–5581 (2003)
A. Frankel, R. Buckman, R.S. Kerbel, Abrogation of taxol-induced G2-M arrest and apoptosis in human ovarian cancer cells grown as multicellular tumor spheroids. Cancer Res. 57, 2388–2393 (1997)
Q. Cai, L. Yan, Y. Xu, Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 34, 3315–3324 (2015)
A. Tajbakhsh, M. Rivandi, S. Abedini, A. Pasdar, A. Sahebkar, Regulators and mechanisms of anoikis in triple-negative breast cancer (TNBC): A review. Crit. Rev. Oncol. Hematol. 140, 17–27 (2019)
K.W. Cheng, J.P. Lahad, W.-l. Kuo, A. Lapuk, K. Yamada, N. Auersperg, J. Liu, K. Smith-McCune, K.H. Lu, D. Fishman, J.W. Gray, G.B. Mills, The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 10, 1251 (2004)
S. Salceda, T. Tang, M. Kmet, A. Munteanu, M. Ghosh, R. Macina, W. Liu, G. Pilkington, J. Papkoff, The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res. 306, 128–141 (2005)
C.A. Witz, I.A. Montoya-Rodriguez, S. Cho, V.E. Centonze, L.F. Bonewald, R.S. Schenken, Composition of the extracellular matrix of the peritoneum. J. Soc. Gynecol. Investig. 8, 299–304 (2001)
K. Sawada, A.K. Mitra, A.R. Radjabi, V. Bhaskar, E.O. Kistner, M. Tretiakova, S. Jagadeeswaran, A. Montag, A. Becker, H.A. Kenny, M.E. Peter, V. Ramakrishnan, S.D. Yamada, E. Lengyel, Loss of E-Cadherin promotes ovarian cancer metastasis via α(5)-integrin, which is a therapeutic target. Cancer Res. 68, 2329–2339 (2008)
A.A. Kamat, M. Fletcher, L.M. Gruman, P. Mueller, A. Lopez, C.N. Landen Jr., L. Han, D.M. Gershenson, A.K. Sood, The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin. Cancer Res. 12, 1707–1714 (2006)
M. Egeblad, Z. Werb, New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174 (2002)
H.A. Kenny, S. Kaur, L.M. Coussens, E. Lengyel, The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Invest. 118, 1367–1379 (2008)
A. Rump, Y. Morikawa, M. Tanaka, S. Minami, N. Umesaki, M. Takeuchi, A. Miyajima, Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 279, 9190–9198 (2004)
X. Fang, M. Schummer, M. Mao, S. Yu, F.H. Tabassam, R. Swaby, Y. Hasegawa, J.L. Tanyi, R. LaPushin, A. Eder, R. Jaffe, J. Erickson, G.B. Mills, Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta 1582, 257–264 (2002)
D.A. Fishman, Y. Liu, S.M. Ellerbroek, M.S. Stack, Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 61, 3194–3199 (2001)
T.B. Pustilnik, V. Estrella, J.R. Wiener, M. Mao, A. Eder, M.A. Watt, R.C. Bast Jr., G.B. Mills, Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin. Cancer Res. 5, 3704–3710 (1999)
D. Bian, S. Su, C. Mahanivong, R.K. Cheng, Q. Han, Z.K. Pan, P. Sun, S. Huang, Lysophosphatidic acid stimulates ovarian cancer cell migration via a Ras-MEK kinase 1 pathway. Cancer Res. 64, 4209–4217 (2004)
R. Agarwal, T. D'Souza, P.J. Morin, Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 65, 7378–7385 (2005)
T. Yagyu, H. Kobayashi, H. Matsuzaki, K. Wakahara, T. Kondo, N. Kurita, H. Sekino, K. Inagaki, Enhanced spontaneous metastasis in bikunin-deficient mice. Int. J. Cancer 118, 2322–2328 (2006)
S. Cai, P. Zhang, S. Dong, L. Li, J. Cai, M. Xu, Downregulation of SPINK13 Promotes Metastasis by Regulating uPA in Ovarian Cancer Cells. Cell. Physiol. Biochem. 45, 1061–1071 (2018)
X.Y. Zhang, R. Pettengell, N. Nasiri, V. Kalia, A.G. Dalgleish, D.P. Barton, Characteristics and growth patterns of human peritoneal mesothelial cells: comparison between advanced epithelial ovarian cancer and non-ovarian cancer sources. J. Soc. Gynecol. Investig. 6, 333–340 (1999)
A.K. Mitra, C.Y. Chiang, P. Tiwari, S. Tomar, K.M. Watters, M.E. Peter, E. Lengyel, Microenvironment-induced downregulation of miR-193b drives ovarian cancer metastasis. Oncogene 34, 5923–5932 (2015)
S. Tomar, J.P. Plotnik, J. Haley, J. Scantland, S. Dasari, Z. Sheikh, R. Emerson, D. Lenz, P.C. Hollenhorst, A.K. Mitra, ETS1 induction by the microenvironment promotes ovarian cancer metastasis through focal adhesion kinase. Cancer Lett. 414, 190–204 (2018)
R.S. Freedman, M. Deavers, J. Liu, E. Wang, Peritoneal inflammation – A microenvironment for Epithelial Ovarian Cancer (EOC). J. Transl. Med. 2, 23 (2004)
A. Feki, P. Berardi, G. Bellingan, A. Major, K.H. Krause, P. Petignat, R. Zehra, S. Pervaiz, I. Irminger-Finger, Dissemination of intraperitoneal ovarian cancer: Discussion of mechanisms and demonstration of lymphatic spreading in ovarian cancer model. Crit. Rev. Oncol. Hematol. 72, 1–9 (2009)
G. Balbi, M.A. Manganaro, A. Monteverde, I. Landino, C. Franzese, F. Gioia, Ovarian cancer: lymph node metastases. European J. Gynaecol. Oncol. 30, 289–291 (2009)
S.S. Chen, Survival of ovarian carcinoma with or without lymph node metastasis. Gynecol. Oncol. 27, 368–372 (1987)
C. Bachmann, R. Bachmann, F. Fend, D. Wallwiener, Incidence and impact of lymph node metastases in advanced ovarian cancer: Implications for surgical treatment. J. Cancer 7, 2241–2246 (2016)
K. Matsuo, T.B. Sheridan, K. Yoshino, T. Miyake, K.E. Hew, D.D. Im, N.B. Rosenshein, S. Mabuchi, T. Enomoto, T. Kimura, A.K. Sood, L.D. Roman, Significance of lymphovascular space invasion in epithelial ovarian cancer. Cancer Med. 1, 156–164 (2012)
M. Chen, Y. Jin, Y. Bi, Y. Li, Y. Shan, L. Pan, Prognostic significance of lymphovascular space invasion in epithelial ovarian cancer. J. Cancer 6, 412–419 (2015)
P. Wimberger, S. Hauch, M. Lustig, R. Kimmig, S. Kasimir-Bauer, Detection and molecular profiling of circulating tumor cells in patients with primary ovarian cancer. Cancer Res. 68, 965 (2008)
K.G. Phillips, C.R. Velasco, J. Li, A. Kolatkar, M. Luttgen, K. Bethel, B. Duggan, P. Kuhn, O.J. McCarty, Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front. Oncol. 2, 72 (2012)
L. Cui, J. Kwong, C.C. Wang, Prognostic value of circulating tumor cells and disseminated tumor cells in patients with ovarian cancer: a systematic review and meta-analysis. J Ovarian Res. 8, 38 (2015)
M.C. Lim, S. Kang, K.S. Lee, S.S. Han, S.J. Park, S.S. Seo, S.Y. Park, The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol. Oncol. 112, 28–34 (2009)
L.G. Coffman, D. Burgos-Ojeda, R. Wu, K. Cho, S. Bai, R.J. Buckanovich, New models of hematogenous ovarian cancer metastasis demonstrate preferential spread to the ovary and a requirement for the ovary for abdominal dissemination. Transl. Res. 175, 92–102.e2 (2016)
P.C. Bailey, S.S. Martin, Insights on CTC biology and clinical impact emerging from advances in capture technology. Cells 8, pii: E553 (2019)
M. Yousefi, P. Ghaffari, R. Nosrati, S. Dehghani, A. Salmaninejad, Y.J. Abarghan, S.H. Ghaffari, Prognostic and therapeutic significance of circulating tumor cells in patients with lung cancer. Cell Oncol. 43, 31–49 (2020)
S.A. Joosse, T.M. Gorges, K. Pantel, Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 7, 1–11 (2015)
M. Yousefi, R. Nosrati, A. Salmaninejad, S. Dehghani, A. Shahryari, A. Saberi, Organ-specific metastasis of breast cancer: molecular and cellular mechanisms underlying lung metastasis. Cell Oncol. 41, 123–140 (2018)
Y. Wang, Y. Zhou, Z. Hu, The functions of circulating tumor cells in early diagnosis and surveillance during cancer advancement. J. Trans. Intern Med. 5, 135–138 (2017)
C. Paoletti, D.F. Hayes, Circulating tumor cells. Adv. Exp. Med. Biol. 882, 235–258 (2016)
M. Cristofanilli, G.T. Budd, M.J. Ellis, A. Stopeck, J. Matera, M.C. Miller, J.M. Reuben, G.V. Doyle, W.J. Allard, L.W. Terstappen, D.F. Hayes, Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004)
M.C. Miller, G.V. Doyle, L.W. Terstappen, Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 617421 (2010)
D.T. Miyamoto, L.V. Sequist, R.J. Lee, Circulating tumour cells-monitoring treatment response in prostate cancer. Nat. Rev. Clin. Oncol. 11, 401–412 (2014)
B. Aktas, S. Kasimir-Bauer, M. Heubner, R. Kimmig, P. Wimberger, Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int. J. Gynecol. Cancer 21, 822–830 (2011)
E. Obermayr, D.C. Castillo-Tong, D. Pils, P. Speiser, I. Braicu, T. Van Gorp, S. Mahner, J. Sehouli, I. Vergote, R. Zeillinger, Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance -- a study of the OVCAD consortium. Gynecol. Oncol. 128, 15–21 (2013)
M. Sang, X. Wu, X. Fan, M. Sang, X. Zhou, N. Zhou, Multiple MAGE-A genes as surveillance marker for the detection of circulating tumor cells in patients with ovarian cancer. Biomarkers 19, 34–42 (2014)
A.K. Mitra, Ovarian cancer metastasis: a unique mechanism of dissemination (InTech, Tumor Metastasis, 2016)
D. Hanahan, L.M. Coussens, Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012)
P. Nilendu, S. C. Sarode, D. Jahagirdar, I. Tandon, S. Patil, G. S. Sarode, J. K. Pal, N. K. Sharma. Mutual concessions and compromises between stromal cells and cancer cells: driving tumor development and drug resistance. Cell. Oncol. 41, 353–67 (2018)
M.A. Swartz, N. Iida, E.W. Roberts, S. Sangaletti, M.H. Wong, F.E. Yull, L.M. Coussens, Y.A. DeClerck, Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 72, 2473–2480 (2012)
A. Ghoneum, H. Afify, Z. Salih, M. Kelly, N. Said. Role of tumor microenvironment in the pathobiology of ovarian cancer: Insights and therapeutic opportunities. Cancer Med. 10, 5047-5056 (2018)
J.A. Joyce, Therapeutic targeting of the tumor microenvironment. Cancer Cell 7, 513–520 (2005)
P. Cirri, P. Chiarugi, Cancer associated fibroblasts: the dark side of the coin. Am. J. Cancer Res. 1, 482–497 (2011)
N. Eiro, L. Gonzalez, A. Martinez-Ordonez, B. Fernandez-Garcia, L. O. Gonzalez, S. Cid, F. Dominguez, R. Perez-Fernandez, F. J. Vizoso. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell. Oncol. 41, 369–78 (2018)
A. Orimo, R.A. Weinberg, Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5, 1597–1601 (2006)
I.G. Schauer, A.K. Sood, S. Mok, J. Liu, Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia (New York, NY) 13, 393–405 (2011)
R. Kalluri, M. Zeisberg, Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006)
M. Yanez-Mo, E. Lara-Pezzi, R. Selgas, M. Ramirez-Huesca, C. Dominguez-Jimenez, J.A. Jimenez-Heffernan, A. Aguilera, J.A. Sanchez-Tomero, M.A. Bajo, V. Alvarez, M.A. Castro, G. del Peso, A. Cirujeda, C. Gamallo, F. Sanchez-Madrid, M. Lopez-Cabrera, Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 348, 403–413 (2003)
P. Sandoval, J.A. Jimenez-Heffernan, A. Rynne-Vidal, M.L. Perez-Lozano, A. Gilsanz, V. Ruiz-Carpio, R. Reyes, J. Garcia-Bordas, K. Stamatakis, J. Dotor, P.L. Majano, M. Fresno, C. Cabanas, M. Lopez-Cabrera, Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J. Pathol. 231, 517–531 (2013)
A. Rynne-Vidal, C. L. Au-Yeung, J. A. Jimenez-Heffernan, M. L. Perez-Lozano, L. Cremades-Jimeno, C. Barcena, I. Cristobal-Garcia, C. Fernandez-Chacon, T. L. Yeung, S. C. Mok, P. Sandoval. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J Pathol. 242, 140–51 (2017)
B. Dirat, L. Bochet, M. Dabek, D. Daviaud, S. Dauvillier, B. Majed, Y.Y. Wang, A. Meulle, B. Salles, S. Le Gonidec, I. Garrido, G. Escourrou, P. Valet, C. Muller, Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71, 2455–2465 (2011)
L. Bochet, C. Lehuede, S. Dauvillier, Y.Y. Wang, B. Dirat, V. Laurent, C. Dray, R. Guiet, I. Maridonneau-Parini, S. Le Gonidec, B. Couderc, G. Escourrou, P. Valet, C. Muller, Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668 (2013)
E. Zoico, E. Darra, V. Rizzatti, S. Budui, G. Franceschetti, G. Mazzali, A.P. Rossi, F. Fantin, M. Menegazzi, S. Cinti, M. Zamboni, Adipocytes WNT5a mediated dedifferentiation: a possible target in pancreatic cancer microenvironment. Oncotarget 7, 20223–20235 (2016)
H.M. Lawler, C.M. Underkofler, P.A. Kern, C. Erickson, B. Bredbeck, N. Rasouli, Adipose tissue hypoxia, inflammation, and fibrosis in obese insulin-sensitive and obese insulin-resistant subjects. J. Clin. Endocrinol. Metab. 101, 1422–1428 (2016)
J. Cai, H. Tang, L. Xu, X. Wang, C. Yang, S. Ruan, J. Guo, S. Hu, Z. Wang, Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis 33, 20–29 (2012)
A. Ghoneum, H. Afify, Z. Salih, M. Kelly, N. Said, Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget 9, 22832–22849 (2018)
T. Dong, D. Yang, R. Li, L. Zhang, H. Zhao, Y. Shen, X. Zhang, B. Kong, L. Wang, PGRN promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Exp. Mol. Pathol. 100, 17–25 (2016)
M. Di Francesco, S. D'Ascenzo, M.G. Palmerini, G. Macchiarelli, G. Carta, V. Dolo, Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior AU - Giusti. Ilaria. Cancer Biology & Therapy 19, 722–734 (2018)
V. Sundararajan, F.H. Sarkar, T.S. Ramasamy, The multifaceted role of exosomes in cancer progression: diagnostic and therapeutic implications [corrected]. Cell. Oncol. 41, 223–252 (2018)
A.K. Mitra, M. Zillhardt, Y. Hua, P. Tiwari, A.E. Murmann, M.E. Peter, E. Lengyel, MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2, 1100–1108 (2012)
Y. Zhang, H. Tang, J. Cai, T. Zhang, J. Guo, D. Feng, Z. Wang, Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 303, 47–55 (2011)
S. Liekens, D. Schols, S. Hatse, CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr. Pharm. Des. 16, 3903–3920 (2010)
T.L. Yeung, C.S. Leung, K.K. Wong, G. Samimi, M.S. Thompson, J. Liu, T.M. Zaid, S. Ghosh, M.J. Birrer, S.C. Mok, TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 73, 5016–5028 (2013)
A. Salmaninejad, S.F. Valilou, A. Soltani, S. Ahmadi, Y.J. Abarghan, R.J. Rosengren, A. Sahebkar, Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell. Oncol. 42, 591–608 (2019)
A. Mantovani, P. Allavena, A. Sica, F. Balkwill, Cancer-related inflammation. Nature 454, 436–444 (2008)
K. Kawamura, Y. Komohara, K. Takaishi, H. Katabuchi, M. Takeya, Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol. Int. 59, 300–305 (2009)
E. Schutyser, S. Struyf, P. Proost, G. Opdenakker, G. Laureys, B. Verhasselt, L. Peperstraete, I. Van de Putte, A. Saccani, P. Allavena, A. Mantovani, J. Van Damme, Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J. Biol. Chem. 277, 24584–24593 (2002)
L.S. Ojalvo, C.A. Whittaker, J.S. Condeelis, J.W. Pollard, Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J. Immunol. 184, 702–712 (2010)
M. Torroella-Kouri, R. Silvera, D. Rodriguez, R. Caso, A. Shatry, S. Opiela, D. Ilkovitch, R.A. Schwendener, V. Iragavarapu-Charyulu, Y. Cardentey, N. Strbo, D.M. Lopez, Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 69, 4800–4809 (2009)
S.K. Biswas, L. Gangi, S. Paul, T. Schioppa, A. Saccani, M. Sironi, B. Bottazzi, A. Doni, B. Vincenzo, F. Pasqualini, L. Vago, M. Nebuloni, A. Mantovani, A. Sica, A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107, 2112–2122 (2006)
L.S. Ojalvo, W. King, D. Cox, J.W. Pollard, High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am. J. Pathol. 174, 1048–1064 (2009)
B.Z. Qian, J.W. Pollard, Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010)
D. Hambardzumyan, D.H. Gutmann, H. Kettenmann, The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20 (2015)
V. Kumar, P. Cheng, T. Condamine, S. Mony, L.R. Languino, J.C. McCaffrey, N. Hockstein, M. Guarino, G. Masters, E. Penman, F. Denstman, X. Xu, D.C. Altieri, H. Du, C. Yan, D.I. Gabrilovich, CD45 phosphatase inhibits STAT3 transcriptionfFactor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity 44, 303–315 (2016)
A. Mantovani, F. Marchesi, A. Malesci, L. Laghi, P. Allavena, Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017)
S.K. Biswas, A. Mantovani, Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010)
R. Clark, V. Krishnan, M. Schoof, I. Rodriguez, B. Theriault, M. Chekmareva, C. Rinker-Schaeffer, Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am. J. Pathol. 183, 576–591 (2013)
T.M. Robinson-Smith, I. Isaacsohn, C.A. Mercer, M. Zhou, N. Van Rooijen, N. Husseinzadeh, M.M. McFarland-Mancini, A.F. Drew, Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 67, 5708–5716 (2007)
J. Liu, X. Geng, Y. Li, Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour Biol. 37, 5715–5726 (2016)
X. Yuan, J. Zhang, D. Li, Y. Mao, F. Mo, W. Du, X. Ma, Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 147, 181–187 (2017)
L.S. Ojalvo, E.D. Thompson, T.L. Wang, A.K. Meeker, I.M. Shih, A.N. Fader, A. Cimino-Mathews, L.A. Emens, Tumor-associated macrophages and the tumor immune microenvironment of primary and recurrent epithelial ovarian cancer. Hum. Pathol. 74, 135–147 (2018)
S. Huang, M. Van Arsdall, S. Tedjarati, M. McCarty, W. Wu, R. Langley, I.J. Fidler, Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J. Natl. Cancer Inst. 94, 1134–1142 (2002)
X. Wang, M. Deavers, R. Patenia, R.L. Bassett Jr., P. Mueller, Q. Ma, E. Wang, R.S. Freedman, Monocyte/macrophage and T-cell infiltrates in peritoneum of patients with ovarian cancer or benign pelvic disease. J. Transl. Med. 4, 30 (2006)
S.F. Schoppmann, A. Fenzl, K. Nagy, S. Unger, G. Bayer, S. Geleff, M. Gnant, R. Horvat, R. Jakesz, P. Birner, VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery 139, 839–846 (2006)
L. Liu, X. Wang, X. Li, X. Wu, M. Tang, X. Wang, Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol. Rep. 39, 818–826 (2018)
N. Nishida, H. Yano, T. Nishida, T. Kamura, M. Kojiro, Angiogenesis in cancer. Vasc. Health Risk Manag. 2, 213–219 (2006)
M. Rajabi, S.A. Mousa, The Role of Angiogenesis in Cancer Treatment. Biomedicines 5, 34 (2017)
T. Tonini, F. Rossi, P.P. Claudio, Molecular basis of angiogenesis and cancer. Oncogene 22, 6549–6556 (2003)
A.K. Olsson, A. Dimberg, J. Kreuger, L. Claesson-Welsh, VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 (2006)
S. Dehghani, R. Nosrati, M. Yousefi, A. Nezami, F. Soltani, S.M. Taghdisi, K. Abnous, M. Alibolandi, M. Ramezani, Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): A review. Biosens. Bioelectron. 110, 23–37 (2018)
M.J. Birrer, M.E. Johnson, K. Hao, K.K. Wong, D.C. Park, A. Bell, W.R. Welch, R.S. Berkowitz, S.C. Mok, Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. J. Clin. Oncol. 25, 2281–2287 (2007)
T.M. Zaid, T.L. Yeung, M.S. Thompson, C.S. Leung, T. Harding, N.N. Co, R.S. Schmandt, S.Y. Kwan, C. Rodriguez-Aguay, G. Lopez-Berestein, A.K. Sood, K.K. Wong, M.J. Birrer, S.C. Mok, Identification of FGFR4 as a potential therapeutic target for advanced-stage, high-grade serous ovarian cancer. Clin. Cancer Res. 19, 809–820 (2013)
W. Wei, S.C. Mok, E. Oliva, S.H. Kim, G. Mohapatra, M.J. Birrer, FGF18 as a prognostic and therapeutic biomarker in ovarian cancer. J. Clin. Invest. 123, 4435–4448 (2013)
L. Hu, L. Cong, Fibroblast growth factor 19 is correlated with an unfavorable prognosis and promotes progression by activating fibroblast growth factor receptor 4 in advanced-stage serous ovarian cancer. Oncol. Rep. 34, 2683–2691 (2015)
X. Wang, Q. Zhu, Y. Lin, L. Wu, X. Wu, K. Wang, Q. He, C. Xu, X. Wan, X. Wang, Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. Br. J. Cancer 117, 1371–1382 (2017)
J. Yang, Y. Wang, Z. Zeng, L. Qiao, L. Zhuang, Q. Gao, D. Ma, X. Huang, Smad4 deletion in blood vessel endothelial cells promotes ovarian cancer metastasis. Int. J. Oncol. 50, 1693–1700 (2017)
M. Yin, H.J. Zhou, J. Zhang, C. Lin, H. Li, X. Li, Y. Li, H. Zhang, D.G. Breckenridge, W. Ji, W. Min, ASK1-dependent endothelial cell activation is critical in ovarian cancer growth and metastasis. JCI Insight 2 (2017)
J. Hoarau-Vechot, C. Touboul, N. Halabi, M. Blot-Dupin, R. Lis, C. Abi Khalil, S. Rafii, A. Rafii, J. Pasquier, Akt-activated endothelium promotes ovarian cancer proliferation through notch activation. J. Transl. Med. 17, 194 (2019)
A. Nowicka, F.C. Marini, T.N. Solley, P.B. Elizondo, Y. Zhang, H.J. Sharp, R. Broaddus, M. Kolonin, S.C. Mok, M.S. Thompson, W.A. Woodward, K. Lu, B. Salimian, D. Nagrath, A.H. Klopp, Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One 8, e81859 (2013)
P. Sartipy, D.J. Loskutoff, Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 100, 7265–7270 (2003)
G.K. Reeves, K. Pirie, V. Beral, J. Green, E. Spencer, D. Bull, Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Bmj 335, 1134 (2007)
A. Ghasemi, J. Saeidi, M. Azimi-Nejad, S. I. Hashemy. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell. Oncol. 42, 243–60 (2019)
A.G. Renehan, M. Tyson, M. Egger, R.F. Heller, M. Zwahlen, Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008)
E.E. Calle, C. Rodriguez, K. Walker-Thurmond, M.J. Thun, Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New Eng. J. Med. 348, 1625–1638 (2003)
E.S. Trombetta, I. Mellman, Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23, 975–1028 (2005)
F. Veglia, D.I. Gabrilovich, Dendritic cells in cancer: the role revisited. Curr. Opin. Immunol. 45, 43–51 (2017)
E. Segura, S. Amigorena, Inflammatory dendritic cells in mice and humans. Trends Immunol. 34, 440–445 (2013)
E. Daro, B. Pulendran, K. Brasel, M. Teepe, D. Pettit, D.H. Lynch, D. Vremec, L. Robb, K. Shortman, H.J. McKenna, C.R. Maliszewski, E. Maraskovsky, Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 165, 49–58 (2000)
S. Menezes, D. Melandri, G. Anselmi, T. Perchet, J. Loschko, J. Dubrot, R. Patel, E.L. Gautier, S. Hugues, M.P. Longhi, J.Y. Henry, S.A. Quezada, G. Lauvau, A.M. Lennon-Dumenil, E. Gutierrez-Martinez, A. Bessis, E. Gomez-Perdiguero, C.E. Jacome-Galarza, H. Garner, F. Geissmann, R. Golub, M.C. Nussenzweig, P. Guermonprez, The heterogeneity of Ly6C(hi) monocytes controls their differentiation into iNOS(+) macrophages or monocyte-derived dendritic cells. Immunity 45, 1205–1218 (2016)
S. Kuhn, E.J. Hyde, J. Yang, F.J. Rich, J.L. Harper, J.R. Kirman, F. Ronchese, Increased numbers of monocyte-derived dendritic cells during successful tumor immunotherapy with immune-activating agents. J. Immunol. 191, 1984–1992 (2013)
E. Segura, M. Touzot, A. Bohineust, A. Cappuccio, G. Chiocchia, A. Hosmalin, M. Dalod, V. Soumelis, S. Amigorena, Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38, 336–348 (2013)
B. Ruffell, D. Chang-Strachan, V. Chan, A. Rosenbusch, C.M. Ho, N. Pryer, D. Daniel, E.S. Hwang, H.S. Rugo, L.M. Coussens, Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 26, 623–637 (2014)
A. Salmaninejad, S.F. Valilou, A.G. Shabgah, S. Aslani, M. Alimardani, A. Pasdar, A. Sahebkar, PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 234, 16824–16837 (2019)
H. Salmon, J. Idoyaga, A. Rahman, M. Leboeuf, R. Remark, S. Jordan, M. Casanova-Acebes, M. Khudoynazarova, J. Agudo, N. Tung, S. Chakarov, C. Rivera, B. Hogstad, M. Bosenberg, D. Hashimoto, S. Gnjatic, N. Bhardwaj, A.K. Palucka, B.D. Brown, J. Brody, F. Ginhoux, M. Merad, Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016)
D.L. Herber, W. Cao, Y. Nefedova, S.V. Novitskiy, S. Nagaraj, V.A. Tyurin, A. Corzo, H.I. Cho, E. Celis, B. Lennox, S.C. Knight, T. Padhya, T.V. McCaffrey, J.C. McCaffrey, S. Antonia, M. Fishman, R.L. Ferris, V.E. Kagan, D.I. Gabrilovich, Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 16, 880–886 (2010)
J.R. Cubillos-Ruiz, P.C. Silberman, M.R. Rutkowski, S. Chopra, A. Perales-Puchalt, M. Song, S. Zhang, S.E. Bettigole, D. Gupta, K. Holcomb, L.H. Ellenson, T. Caputo, A.-H. Lee, J.R. Conejo-Garcia, L.H. Glimcher, ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538 (2015)
K. Dass, A. Ahmad, A.S. Azmi, S.H. Sarkar, F.H. Sarkar, Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 34, 122–136 (2008)
L. Wang, M.C. Madigan, H. Chen, F. Liu, K.I. Patterson, J. Beretov, P.M. O'Brien, Y. Li, Expression of urokinase plasminogen activator and its receptor in advanced epithelial ovarian cancer patients. Gynecol. Oncol. 114, 265–272 (2009)
J. Dorn, N. Harbeck, R. Kates, A. Gkazepis, A. Scorilas, A. Soosaipillai, E. Diamandis, M. Kiechle, B. Schmalfeldt, M. Schmitt, Impact of expression differences of kallikrein-related peptidases and of uPA and PAI-1 between primary tumor and omentum metastasis in advanced ovarian cancer. Ann. Oncol. 22, 877–883 (2011)
C. Alberti, P. Pinciroli, B. Valeri, R. Ferri, A. Ditto, K. Umezawa, M. Sensi, S. Canevari, A. Tomassetti, Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene 31, 4139–4149 (2012)
P.A. van Dam, A. Coelho, C. Rolfo, Is there a role for urokinase-type plasminogen activator inhibitors as maintenance therapy in patients with ovarian cancer? Eur. J. Surg. Oncol. 43, 252–257 (2017)
B. Schmalfeldt, D. Prechtel, K. Harting, K. Spathe, S. Rutke, E. Konik, R. Fridman, U. Berger, M. Schmitt, W. Kuhn, E. Lengyel, Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin. Cancer Res. 7, 2396–2404 (2001)
M. Maatta, M. Santala, Y. Soini, A. Talvensaari-Mattila, T. Turpeenniemi-Hujanen, Matrix metalloproteinases 2 and 9 and their tissue inhibitors in low malignant potential ovarian tumors. Tumour Biol. 25, 188–192 (2004)
S. Sillanpaa, M. Anttila, K. Voutilainen, K. Ropponen, T. Turpeenniemi-Hujanen, U. Puistola, R. Tammi, M. Tammi, R. Sironen, S. Saarikoski, V.M. Kosma, Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol. Oncol. 104, 296–303 (2007)
H. Nishikawa, Y. Ozaki, T. Nakanishi, K. Blomgren, T. Tada, A. Arakawa, K. Suzumori, The role of cathepsin B and cystatin C in the mechanisms of invasion by ovarian cancer. Gynecol. Oncol. 92, 881–886 (2004)
J.L. Brun, A. Cortez, F. Commo, S. Uzan, R. Rouzier, E. Darai, Serous and mucinous ovarian tumors express different profiles of MMP-2, −7, −9, MT1-MMP, and TIMP-1 and -2. Int. J. Oncol. 33, 1239–1246 (2008)
Z.S. Wu, Q. Wu, J.H. Yang, H.Q. Wang, X.D. Ding, F. Yang, X.C. Xu, Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int. J. Cancer 122, 2050–2056 (2008)
L.S. Downs Jr., P.H. Lima, R.L. Bliss, C.H. Blomquist, Cathepsins B and D activity and activity ratios in normal ovaries, benign ovarian neoplasms, and epithelial ovarian cancer. J. Soc. Gynecol. Investig. 12, 539–544 (2005)
Q. Pan, S. Yang, Y. Wei, F. Sun, Z. Li, SP1 acts as a key factor, contributes to upregulation of ADAM23 expression under serum deprivation. Biochem. Biophys. Res. Commun. 401, 306–312 (2010)
C. Bret, D. Hose, T. Reme, A. Kassambara, A. Seckinger, T. Meissner, J.F. Schved, T. Kanouni, H. Goldschmidt, B. Klein, Gene expression profile of ADAMs and ADAMTSs metalloproteinases in normal and malignant plasma cells and in the bone marrow environment. Exp. Hematol. 39, 546–57.e8 (2011)
J. Lin, J. Luo, C. Redies, Differential regional expression of multiple ADAMs during feather bud formation. Dev. Dyn. 240, 2142–2152 (2011)
R. Ma, Z. Tang, K. Sun, X. Ye, H. Cheng, X. Chang, H. Cui, Low levels of ADAM23 expression in epithelial ovarian cancer are associated with poor survival. Pathol. Res. Pract. 214, 1115–1122 (2018)
G. Pampalakis, G. Sotiropoulou, Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim. Biophys. Acta 1776, 22–31 (2007)
A. Psyrri, P. Kountourakis, A. Scorilas, S. Markakis, R. Camp, D. Kowalski, E.P. Diamandis, M.A. Dimopoulos, Human tissue kallikrein 7, a novel biomarker for advanced ovarian carcinoma using a novel in situ quantitative method of protein expression. Ann. Oncol. 19, 1271–1277 (2008)
C. Caubet, N. Jonca, M. Brattsand, M. Guerrin, D. Bernard, R. Schmidt, T. Egelrud, M. Simon, G. Serre, Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 122, 1235–1244 (2004)
Y. Dong, O.L. Tan, D. Loessner, C. Stephens, C. Walpole, G.M. Boyle, P.G. Parsons, J.A. Clements, Kallikrein-related peptidase 7 promotes multicellular aggregation via the alpha(5)beta(1) integrin pathway and paclitaxel chemoresistance in serous epithelial ovarian carcinoma. Cancer Res. 70, 2624–2633 (2010)
Y. Cui, Y. Wang, H. Li, Q. Li, Y. Yu, X. Xu, B. Xu, T. Liu, Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget 7, 34356–34370 (2016)
P. Guo, Z. Zhu, Z. Sun, Z. Wang, X. Zheng, H. Xu, Expression of legumain correlates with prognosis and metastasis in gastric carcinoma. PLoS One 8, e73090 (2013)
Y. Lin, Y. Qiu, C. Xu, Q. Liu, B. Peng, G.F. Kaufmann, X. Chen, B. Lan, C. Wei, D. Lu, Y. Zhang, Y. Guo, Z. Lu, B. Jiang, T.S. Edgington, F. Guo, Functional role of asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion and metastasis. J. Natl. Cancer Inst. 106, dju012 (2014)
M.H. Haugen, K. Boye, J.M. Nesland, S.J. Pettersen, E.V. Egeland, T. Tamhane, K. Brix, G.M. Maelandsmo, K. Flatmark, High expression of the cysteine proteinase legumain in colorectal cancer - implications for therapeutic targeting. Eur. J. Cancer 51, 9–17 (2015)
Q. Zhu, M. Tang, X. Wang, The expression of asparaginyl endopeptidase promotes growth potential in epithelial ovarian cancer. Cancer Biol. Ther. 18, 222–228 (2017)
J. Cheng, M. Su, Y. Jin, Q. Xi, Y. Deng, J. Chen, W. Wang, Y. Chen, L. Chen, N. Shi, G. Mao, Upregulation of SENP3/SMT3IP1 promotes epithelial ovarian cancer progression and forecasts poor prognosis. Tumour Biol. 39, 1010428317694543 (2017)
Y. Klymenko, O. Kim, E. Loughran, J. Yang, R. Lombard, M. Alber, M.S. Stack, Cadherin composition and multicellular aggregate invasion in organotypic models of epithelial ovarian cancer intraperitoneal metastasis. Oncogene 36, 5840–5851 (2017)
V. Azimian-Zavareh, G. Hossein, M. Ebrahimi, Z. Dehghani-Ghobadi, Wnt11 alters integrin and cadherin expression by ovarian cancer spheroids and inhibits tumorigenesis and metastasis. Exp. Cell Res. 369, 90–104 (2018)
X. Li, M. Tang, Q. Zhu, X. Wang, Y. Lin, X. Wang. The exosomal integrin α5β1/AEP complex derived from epithelial ovarian cancer cells promotes peritoneal metastasis through regulating mesothelial cell proliferation and migration. Cell. Oncol. 43, 263-277 (2020)
C.H. Chen, S.H. Wang, C.H. Liu, Y.L. Wu, W.J. Wang, J. Huang, J.S. Hung, I.R. Lai, J.T. Liang, M.C. Huang, beta-1,4-Galactosyltransferase III suppresses beta1 integrin-mediated invasive phenotypes and negatively correlates with metastasis in colorectal cancer. Carcinogenesis 35, 1258–1266 (2014)
Q. Li, S. Liu, B. Lin, L. Yan, Y. Wang, C. Wang, S. Zhang, Expression and correlation of Lewis y antigen and integrins alpha5 and beta1 in ovarian serous and mucinous carcinoma. Int. J. Gynecol. Cancer 20, 1482–1489 (2010)
J.K. Slack-Davis, K.A. Atkins, C. Harrer, E.D. Hershey, M. Conaway, Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 69, 1469–1476 (2009)
W.M. Hsu, M.I. Che, Y.F. Liao, H.H. Chang, C.H. Chen, Y.M. Huang, Y.M. Jeng, J. Huang, M.J. Quon, H. Lee, H.C. Huang, M.C. Huang, B4GALNT3 expression predicts a favorable prognosis and suppresses cell migration and invasion via beta(1) integrin signaling in neuroblastoma. Am. J. Pathol. 179, 1394–1404 (2011)
C.H. Chen, S.W. Wang, C.W. Chen, M.R. Huang, J.S. Hung, H.C. Huang, H.H. Lin, R.J. Chen, M.K. Shyu, M.C. Huang, MUC20 overexpression predicts poor prognosis and enhances EGF-induced malignant phenotypes via activation of the EGFR-STAT3 pathway in endometrial cancer. Gynecol. Oncol. 128, 560–567 (2013)
C.H. Chou, M.J. Huang, C.H. Chen, M.K. Shyu, J. Huang, J.S. Hung, C.S. Huang, M.C. Huang, Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 6, 6123–6135 (2015)
M. Yousefi, S. Dehghani, R. Nosrati, H. Zare, M. Evazalipour, J. Mosafer, B.S. Tehrani, A. Pasdar, A. Mokhtarzadeh, M. Ramezani, Aptasensors as a new sensing technology developed for the detection of MUC1 mucin: A review. Biosens. Bioelectron. 130, 1–19 (2019)
Y.F. He, M.Y. Zhang, X. Wu, X.J. Sun, T. Xu, Q.Z. He, W. Di, High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PLoS One 8, e79769 (2013)
M.P. Ponnusamy, I. Lakshmanan, M. Jain, S. Das, S. Chakraborty, P. Dey, S.K. Batra, MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene 29, 5741–5754 (2010)
C.H. Chen, M.K. Shyu, S.W. Wang, C.H. Chou, M.J. Huang, T.C. Lin, S.T. Chen, H.H. Lin, M.C. Huang, MUC20 promotes aggressive phenotypes of epithelial ovarian cancer cells via activation of the integrin beta1 pathway. Gynecol. Oncol. 140, 131–137 (2016)
T. Motohara, K. Masuda, M. Morotti, Y. Zheng, S. El-Sahhar, K.Y. Chong, N. Wietek, A. Alsaadi, M. Karaminejadranjbar, Z. Hu, M. Artibani, L.S. Gonzalez, H. Katabuchi, H. Saya, A.A. Ahmed, An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 38, 2885–2898 (2019)
R.L. Anderson, T. Balasas, J. Callaghan, R.C. Coombes, J. Evans, J.A. Hall, S. Kinrade, D. Jones, P.S. Jones, R. Jones, J.F. M+*arshall, M.B. Panico, J.A. Shaw, P.S. Steeg, M. Sullivan, W. Tong, A.D. Westwell, J.W.A. Ritchie, U. K. on behalf of the Cancer Research, C. R. C. A. M. W. G. Cancer Therapeutics, A framework for the development of effective anti-metastatic agents. Nature Rev. Clin. Oncol. 16, 185–204 (2019)
U.H. Weidle, F. Birzele, G. Kollmorgen, R. Rueger, Mechanisms and targets involved in dissemination of ovarian cancer. Cancer Genomics-Proteomics 13, 407–423 (2016)
M.H. Vetter, J.L. Hays, Use of targeted therapeutics in epithelial ovarian cancer: a review of current literature and future directions. Clin. Ther. 40, 361–371 (2018)
B.A. Jones, S. Varambally, R.C. Arend, Histone methyltransferase EZH2: a therapeutic target for ovarian Cancer. Mol. Cancer Ther. 17, 591–602 (2018)
A. F. Chambers, I. C. MacDonald, E. E. Schmidt, V. L. Morris, A. C. Groom. Clinical targets for anti-metastasis therapy. Adv. Cancer Res. 79, 91-121. (2000)
J.O. van Baal, C.J. van Noorden, R. Nieuwland, K.K. Van de Vijver, A. Sturk, W.J. van Driel, G.G. Kenter, C.A. Lok, Development of peritoneal carcinomatosis in epithelial ovarian cancer: a review. J. Histochem. Cytochem. 66, 67–83 (2018)
G.-T. Park, K.-C. Choi, Advanced new strategies for metastatic cancer treatment by therapeutic stem cells and oncolytic virotherapy. Oncotarget 7, 58684 (2016)
V. Conteduca, B. Kopf, S.L. Burgio, E. Bianchi, D. Amadori, U. De Giorgi, The emerging role of anti-angiogenic therapy in ovarian cancer. Int. J. Oncol. 44, 1417–1424 (2014)
M. Barbolina, Molecular Mechanisms Regulating Organ-Specific Metastases in Epithelial Ovarian Carcinoma. Cancers 10, 444 (2018)
Á. Áyen, Y. Jimenez Martinez, J. Marchal, H. Boulaiz, Recent Progress in gene therapy for ovarian Cancer. Int. J. Mol. Sci. 19, 1930 (2018)
X. Chen, L. S. Mangala, L. Mooberry, E. Bayraktar, S. K. Dasari, S. Ma, C. Ivan, K. A. Court, C. Rodriguez-Aguayo, R. Bayraktar. Identifying and targeting angiogenesis-related microRNAs in ovarian cancer. Oncogene 38, 6095-61081 (2019)
U.H. Weidle, F. Birzele, G. Kollmorgen, A. Nopora, Potential microRNA-related targets for therapeutic intervention with ovarian cancer metastasis. Cancer Genomics-Proteomics 15, 1–15 (2018)
B. Wang, X. Li, G. Zhao, H. Yan, P. Dong, H. Watari, M. Sims, W. Li, L.M. Pfeffer, Y. Guo, miR-203 inhibits ovarian tumor metastasis by targeting BIRC5 and attenuating the TGFβ pathway. J. Exp. Clin. Cancer Res. 37, 235 (2018)
X. Zhou, Y. Hu, L. Dai, Y. Wang, J. Zhou, W. Wang, W. Di, L. Qiu. MicroRNA-7 inhibits tumor metastasis and reverses epithelial-mesenchymal transition through AKT/ERK1/2 inactivation by targeting EGFR in epithelial ovarian cancer. PLoS One 9, (2014)
M. Lee, E.J. Kim, Y. Cho, S. Kim, H.H. Chung, N.H. Park, Y.-S. Song, Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 145, 361–365 (2017)
T. Fan, Q. Zhao, J.J. Chen, W.T. Chen, M.L. Pearl, Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol. Oncol. 112, 185–191 (2009)
A. Poveda, S.B. Kaye, R. McCormack, S. Wang, T. Parekh, D. Ricci, C.A. Lebedinsky, J.C. Tercero, P. Zintl, B.J. Monk, Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol. Oncol. 122, 567–572 (2011)
J.D. Kuhlmann, P. Wimberger, A. Bankfalvi, T. Keller, S. Schöler, B. Aktas, P. Buderath, S. Hauch, F. Otterbach, R. Kimmig, S. Kasimir-Bauer, <em>ERCC1</em>-Positive circulating tumor cells in the blood of ovarian cancer patients as a predictive biomarker for platinum resistance. Clin. Chem. 60, 1282–1289 (2014)
M.L. Pearl, Q. Zhao, J. Yang, H. Dong, S. Tulley, Q. Zhang, M. Golightly, S. Zucker, W.T. Chen, Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol. Oncol. 134, 581–590 (2014)
G. Gebauer, M.J. Banys-Paluchowski, H. Neubauer, N. Krawczyk, A. Kaczerowski, P. Paluchowski, F. Meier-Stiegen, A. Abdel-Kawi, T.N. Fehm, Clinical relevance of circulating tumor cells in ovarian, fallopian tube and peritoneal cancer. J. Clin. Oncol. 35, e17080–e1708e (2017)
X. Zhang, H. Li, X. Yu, S. Li, Z. Lei, C. Li, Q. Zhang, Q. Han, Y. Li, K. Zhang, Y. Wang, C. Liu, Y. Mao, X. Wang, D.M. Irwin, H. Guo, G. Niu, H. Tan, Analysis of circulating tumor cells in ovarian cancer and their clinical value as a biomarker. Cell. Physiol. Biochem. 48, 1983–1994 (2018)
E. Lou, R.I. Vogel, D. Teoh, S. Hoostal, A. Grad, M. Gerber, M. Monu, T. Łukaszewski, J. Deshpande, M.A. Linden, M.A. Geller, Assessment of circulating tumor cells as a predictive biomarker of histology in women with suspected ovarian cancer. Lab. Med. 49, 134–139 (2018)
L. Zuo, W. Niu, A. Li, Isolation of circulating tumor cells of ovarian cancer by transferrin immunolipid magnetic spheres and its preliminary clinical application. Nano LIFE 09, 1940001 (2019)
Acknowledgments
This study was supported by the Department of Medical Genetics and Molecular Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousefi, M., Dehghani, S., Nosrati, R. et al. Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles. Cell Oncol. 43, 515–538 (2020). https://doi.org/10.1007/s13402-020-00513-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00513-9