Abstract
Purpose
Previously, it has been reported that homeobox A9 (HOXA9) protein expression is downregulated in lung cancer cells, and that its expression is inversely correlated with the metastatic potential of lung cancer cells both in vitro and in vivo. As such, HOXA9 shows therapeutic potential. The development of therapeutic strategies based on this protein is, however, limited due to its poor membrane permeability. To overcome this problem, we developed a system to deliver HOXA9 protein into non-small cell lung cancer (NSCLC) cells.
Methods
First, we constructed a delivery vector expressing polyarginine, a cell-penetrating peptide, as well as HOXA9. The resulting recombinant R10-HOXA9 protein was effectively introduced into A549 and NCI-H1299 NSCLC cells. Next, we examined the roles and molecular mechanisms of recombinant R10-HOXA9 in processes involved in tumor progression. To investigate the therapeutic efficacy of the delivery system, we performed cell motility assays using both in vitro and in vivo experimental models.
Results
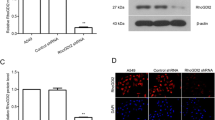
We found that recombinant R10-HOXA9 protein reduced the invasion and migration rate, but not the proliferation rate, of the NSCLC cells tested, both in vitro and in vivo. Treatment of NSCLC cells with recombinant R10-HOXA9 protein led to a significant increase in E-cadherin expression. Conversely, we found that the expression of snail family zinc finger 2 (SLUG), a transcriptional repressor of E-cadherin, was markedly decreased. In an experimental metastatic mouse model, recombinant R10-HOXA9 protein was found to effectively reduce the rate of lung cancer cell motility.
Conclusions
Our data suggest that the developed cell-permeable R10-HOXA9 system may serve as a useful tool to prevent NSCLC cell migration and invasion.




Similar content being viewed by others
References
U. Thorsteinsdottir, A. Mamo, E. Kroon, L. Jerome, J. Bijl, H.J. Lawrence, K. Humphries, G. Sauvageau, Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood 99, 121–129 (2002)
M. Pojo, C.S. Goncalves, A. Xavier-Magalhaes, A.I. Oliveira, T. Goncalves, S. Correia, A.J. Rodrigues, S. Costa, L. Pinto, A.A. Pinto, J.M. Lopes, R.M. Reis, M. Rocha, N. Sousa, B.M. Costa, A transcriptomic signature mediated by HOXA9 promotes human glioblastoma initiation, aggressiveness and resistance to temozolomide. Oncotarget 6, 7657–7674 (2015)
S.Y. Ko, A. Ladanyi, E. Lengyel, H. Naora, Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am. J. Pathol. 184, 271–281 (2014)
P.M. Gilbert, J.K. Mouw, M.A. Unger, J.N. Lakins, M.K. Gbegnon, V.B. Clemmer, M. Benezra, J.D. Licht, N.J. Boudreau, K.K. Tsai, A.L. Welm, M.D. Feldman, B.L. Weber, V.M. Weaver, HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J. Clin. Invest. 120, 1535–1550 (2010)
L. Alvarado-Ruiz, M.G. Martinez-Silva, L.A. Torres-Reyes, P. Pina-Sanchez, P. Ortiz-Lazareno, A. Bravo-Cuellar, A. Aguilar-Lemarroy, L.F. Jave-Suarez, HOXA9 is underexpressed in cervical cancer cells and its restoration decreases proliferation, migration and expression of epithelial-to-mesenchymal transition genes. Asian Pac. J. Cancer Prev. 17, 1037–1047 (2016)
F. Li, L. Dong, X. Qu, K. Li, H. Wang, L. Cheng, H. He, L. Wang, Z. Qi, H. Ren, S. Jin, Down-expression of homeobox A9 promotes cancer cell invasion in human hepatocellular carcinomas. Int. J. Clin. Exp. Pathol. 9, 563–575 (2016)
J.A. Hwang, B.B. Lee, Y. Kim, S.H. Hong, Y.H. Kim, J. Han, Y.M. Shim, C.Y. Yoon, Y.S. Lee, D.H. Kim, HOXA9 inhibits migration of lung cancer cells and its hypermethylation is associated with recurrence in non-small cell lung cancer. Mol. Carcinog. 54, E72–E80 (2015)
J.W. Son, K.J. Jeong, W.S. Jean, S.Y. Park, S. Jheon, H.M. Cho, C.G. Park, H.Y. Lee, J. Kang, Genome-wide combination profiling of DNA copy number and methylation for deciphering biomarkers in non-small cell lung cancer patients. Cancer Lett. 311, 29–37 (2011)
S.H. Hwang, K.U. Kim, J.E. Kim, H.H. Kim, M.K. Lee, C.H. Lee, S.Y. Lee, T. Oh, S. An, Detection of HOXA9 gene methylation in tumor tissues and induced sputum samples from primary lung cancer patients. Clin. Chem. Lab. Med. 49, 699–704 (2011)
S.L. Yu, D.C. Lee, H.A. Sohn, S.Y. Lee, H.S. Jeon, J.H. Lee, C.G. Park, H.Y. Lee, Y.I. Yeom, J.W. Son, Y.S. Yoon, J. Kang, Homeobox A9 directly targeted by miR-196b regulates aggressiveness through nuclear factor-kappa B activity in non-small cell lung cancer cells. Mol. Carcinog. 55, 1915–1926 (2016)
V. Gopal, Bioinspired peptides as versatile nucleic acid delivery platforms. J. Control. Release 167, 323–332 (2013)
H.J. Kim, M.H. Kim, J.T. Kim, W.J. Lee, E. Kim, K.S. Lim, J.K. Kim, Y.I. Yang, K.D. Park, Y.H. Kim, Intracellular transduction of TAT-Hsp27 fusion protein enhancing cell survival and regeneration capacity of cardiac stem cells in acute myocardial infarction. J. Control. Release 215, 55–72 (2015)
H. He, L. Sun, J. Ye, E. Liu, S. Chen, Q. Liang, M.C. Shin, V.C. Yang, Enzyme-triggered, cell penetrating peptide-mediated delivery of anti-tumor agents. J. Control. Release 240, 67–76 (2016)
T. Skotland, T.G. Iversen, M.L. Torgersen, K. Sandvig, Cell-penetrating peptides: possibilities and challenges for drug delivery in vitro and in vivo. Molecules 20, 13313–13323 (2015)
D.A. Mann, A.D. Frankel, Endocytosis and targeting of exogenous HIV-1 tat protein. EMBO J. 10, 1733–1739 (1991)
P.A. Wender, D.J. Mitchell, K. Pattabiraman, E.T. Pelkey, L. Steinman, J.B. Rothbard, The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U. S. A. 97, 13003–13008 (2000)
A. Bolhassani, B.S. Jafarzade, G. Mardani, In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 87, 50–63 (2017)
U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970)
J.H. Cho, Y.M. You, Y. Il Yeom, D.C. Lee, B.K. Kim, M. Won, B.C. Cho, M. Kang, S. Park, S.J. Yang, J.S. Kim, J.A. Kim, K.C. Park, RNF25 promotes gefitinib resistance in EGFR-mutant NSCLC cells by inducing NF-kappaB-mediated ERK reactivation. Cell Death Dis. 9, 587 (2018)
Y. Xia, S. Shen, I.M. Verma, NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2, 823–830 (2014)
G. Guidotti, L. Brambilla, D. Rossi, Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol. Sci. 38, 406–424 (2017)
D.J. Mitchell, D.T. Kim, L. Steinman, C.G. Fathman, J.B. Rothbard, Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 56, 318–325 (2000)
G. Tunnemann, G. Ter-Avetisyan, R.M. Martin, M. Stockl, A. Herrmann, M.C. Cardoso, Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 14, 469–476 (2008)
J.T. Hsieh, J. Zhou, C. Gore, P. Zimmern, R11, a novel cell-permeable peptide, as an intravesical delivery vehicle. BJU Int. 108, 1666–1671 (2011)
T. Zhang, K. Wu, C. Ding, K. Sun, Z. Guan, X. Wang, J.T. Hsieh, D. He, J. Fan, Inhibiting bladder tumor growth with a cell penetrating R11 peptide derived from the p53 C-terminus. Oncotarget 6, 37782–37791 (2015)
W. Wang, N. Zhang, T. Zhao, M. Liu, T. Zhang, D. Li, Inhibition of tumor growth by polyarginine-fused mutant cytosine deaminase. Appl. Biochem. Biotechnol. 175, 1633–1643 (2015)
Y. Du, L. Wang, W. Wang, T. Guo, M. Zhang, P. Zhang, Y. Zhang, K. Wu, A. Li, X. Wang, J. He, J. Fan, Novel application of cell penetrating R11 peptide for diagnosis of bladder cancer. J. Biomed. Nanotechnol. 14, 161–167 (2018)
M. Yousefi, T. Bahrami, A. Salmaninejad, R. Nosrati, P. Ghaffari, S.H. Ghaffari, Lung cancer-associated brain metastasis: Molecular mechanisms and therapeutic options. Cell. Oncol. 40, 419–441 (2017)
Q. Zhang, Y. Zhang, K. Li, H. Wang, H. Li, J. Zheng, A novel strategy to improve the therapeutic efficacy of gemcitabine for non-small cell lung cancer by the tumor-penetrating peptide Irgd. PLoS One 10, e0129865 (2015)
Y. Zhang, J. Yang, M. Ding, L. Li, Z. Lu, Q. Zhang, J. Zheng, Tumor-penetration and antitumor efficacy of cetuximab are enhanced by co-administered iRGD in a murine model of human NSCLC. Oncol. Lett. 12, 3241–3249 (2016)
Z. Duan, C. Chen, J. Qin, Q. Liu, Q. Wang, X. Xu, J. Wang, Cell-penetrating peptide conjugates to enhance the antitumor effect of paclitaxel on drug-resistant lung cancer. Drug Deliv. 24, 752–764 (2017)
N. Mohammad, P. Malvi, A.S. Meena, S.V. Singh, B. Chaube, G. Vannuruswamy, M.J. Kulkarni, M.K. Bhat, Cholesterol depletion by methyl-beta-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol. Cancer 13, 204 (2014)
N. Mohammad, S.V. Singh, P. Malvi, B. Chaube, D. Athavale, M. Vanuopadath, S.S. Nair, B. Nair, M.K. Bhat, Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-beta-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Sci. Rep. 5, 11853 (2015)
Acknowledgments
This work was supported by grants from the National Research Foundation of Korea (NRF-2017R1A2B4003684) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A03015713).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving animal experiments were approved by the Animal Research Ethics Committee of the Korea Research Institute of Bioscience and Biotechnology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Generation of recombinant cell-permeable R10-HOXA9 protein. (a) Construction of the HOXA9 open reading frame or the fusion of ten arginine residues (R10) and the HOXA9 open reading frame using EcoRI and NotI sites in the pET-41a(+) vector. (b) DNA gel electrophoresis after the NotI and EcoRI digestion of subclones. (c) Identification of recombinant C-HOXA9 and R10-HOXA9 expression by IPTG induction using Coomassie blue-stained SDS-PAGE. (d) Recombinant C-HOXA9 and R10-HOXA9 protein were purified using Ni-NTA beads. The purified proteins were identified by Coomassie blue staining (left panel) and immunoblotting using an anti-HOXA9 antibody (right panel). (DOCX 13 kb)
ESM 2
(PDF 342 kb)
Rights and permissions
About this article
Cite this article
Yu, SL., Koo, H., Lee, H.Y. et al. Recombinant cell-permeable HOXA9 protein inhibits NSCLC cell migration and invasion. Cell Oncol. 42, 275–285 (2019). https://doi.org/10.1007/s13402-019-00424-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00424-4