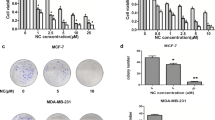
Abstract
Purpose
Triple-negative breast cancers (TNBC) lack expression of three common cell surface receptors, i.e., estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2). Accordingly, TNBCs are associated with fewer treatment options and a relatively poor prognosis. Having screened a National Cancer Institute natural compound library, the purpose of this study was to investigate the bioactivity of compound C4 (Crassin) in TNBC cells.
Methods
Cell viability assays were performed in two TNBC cell lines, MDA-MB-231 and 4T1, following C4 treatment in the presence or absence of the antioxidant N-acetyl-L-cysteine (NAC). Phosphorylation of Akt and ERK was assessed by Western blotting. Apoptosis, necrosis, autophagy, necroptosis, ferroptosis and cytostasis assays were performed to explain viability deficits resulting from C4 exposure.
Results
We found that the viability of the TNBC cells tested decreased in a concentration- and time-dependent fashion following C4 treatment. This decrease coincided with an unexpected increase in the expression of the cell survival effectors pAkt and pERK. In addition, we found that both the decreased cell viability and the increased pAkt/pERK levels could be rescued by the antioxidant NAC, suggesting a central role for reactive oxygen species (ROS) in the mechanism of action of C4. Necrosis, apoptosis, necroptosis and ferroptosis could be ruled out as cell death mechanisms. Instead, we found that C4 induced cytostasis downstream of ROS activation. Finally, we observed a synergistic effect between C4 and the chemotherapeutic drug doxorubicin in TNBC cells.
Conclusions
From our in vitro data we conclude that C4 exerts cytostatic effects on triple-negative breast cancer cells via a pathway involving reactive oxygen species. Its potential value in combination with cytotoxic therapies merits deeper investigation in pre-clinical models.








Similar content being viewed by others
References
R.D. Chacon, M.V. Costanzo, Triple-negative breast cancer. Breast Cancer Res. 12(Suppl 2), S3 (2010)
W.D. Foulkes, I.E. Smith, J.S. Reis-Filho, Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010)
H. Greenlee, Natural products for cancer prevention. Semin. Oncol. Nurs. 28, 29–44 (2012)
A.R. Amin, O. Kucuk, F.R. Khuri, D.M. Shin, Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 27, 2712–2725 (2009)
M.C. Wani, H.L. Taylor, M.E. Wall, P. Coggon, A.T. McPhail, Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93, 2325–2327 (1971)
H. Parekh, H. Simpkins, The transport and binding of taxol. Gen. Pharmacol. 29, 167–172 (1997)
C. Khanna, M. Rosenberg, D.M. Vail, A review of paclitaxel and novel formulations including those suitable for use in dogs. J. Vet. Intern. Med. 29, 1006–1012 (2015)
Q.Q. Yuan, S. Tang, W.B. Song, W.Q. Wang, M. Huang, L.J. Xuan, A.-H. Crassins, Diterpenoids from the roots of croton crassifolius. J. Nat. Prod. 80, 254–260 (2017)
S. Donatello, L. Hudson, D.C. Cottell, A. Blanco, I. Aurrekoetxea, M.J. Shelly, P.A. Dervan, M.R. Kell, M. Stokes, A.D. Hill, A.M. Hopkins, An imbalance in progenitor cell populations reflects tumour progression in breast cancer primary culture models. J. Exp. Clin. Cancer Res. 30, 45 (2011)
S. Nobili, D. Lippi, E. Witort, M. Donnini, L. Bausi, E. Mini, S. Capaccioli, Natural compounds for cancer treatment and prevention. Pharmacol. Res. 59, 365–378 (2009)
N. Hasima, L.I. Aun, M.N. Azmi, A.N. Aziz, E. Thirthagiri, H. Ibrahim, K. Awang, 1′S-1′-acetoxyeugenol acetate: a new chemotherapeutic natural compound against MCF-7 human breast cancer cells. Phytomedicine 17, 935–939 (2010)
A.J. Robles, L. Du, R.H. Cichewicz, S.L. Mooberry, D.N.A. Maximiscin induces damage, activates DNA damage response pathways, and has selective cytotoxic activity against a subtype of triple-negative breast cancer. J. Nat. Prod. 79, 1822–1827 (2016)
E.J. Kim, S.Y. Park, J.Y. Lee, J.H. Park, Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 10, 96 (2010)
H. Yamaguchi, H.G. Wang, The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20, 7779–7786 (2001)
G. Song, G. Ouyang, S. Bao, The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 9, 59–71 (2005)
S.R. Datta, A. Brunet, M.E. Greenberg, Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999)
A. Brunet, A. Bonni, M.J. Zigmond, M.Z. Lin, P. Juo, L.S. Hu, M.J. Anderson, K.C. Arden, J. Blenis, M.E. Greenberg, Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999)
I. Dolado, A.R. Nebreda, AKT and oxidative stress team up to kill cancer cells. Cancer Cell 14, 427–429 (2008)
Y.Q. Hou, Y. Yao, Y.L. Bao, Z.B. Song, C. Yang, X.L. Gao, W.J. Zhang, L.G. Sun, C.L. Yu, Y.X. Huang, G.N. Wang, Y.X. Li, Juglanthraquinone C induces intracellular ROS increase and apoptosis by activating the Akt/foxo signal pathway in HCC cells. Oxidative Med. Cell. Longev. 2016, 4941623 (2016)
Q. Liu, J. Qiu, M. Liang, J. Golinski, K. van Leyen, J.E. Jung, Z. You, E.H. Lo, A. Degterev, M.J. Whalen, Akt and mTOR mediate programmed necrosis in neurons. Cell Death Dis. 5, e1084 (2014)
C. Peyssonnaux, A. Eychene, The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 93, 53–62 (2001)
D.G. Kirsch, A. Doseff, B.N. Chau, D.S. Lim, N.C. de Souza-Pinto, R. Hansford, M.B. Kastan, Y.A. Lazebnik, J.M. Hardwick, Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 274, 21155–21161 (1999)
A.G. Porter, R.U. Janicke, Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104 (1999)
S.J. Dixon, B.R. Stockwell, The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014)
Y. Xie, W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, R. Kang, D. Tang, Ferroptosis: process and function. Cell Death Differ. 23, 369–379 (2016)
D.E. Christofferson, J. Yuan, Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22, 263–268 (2010)
S. Fulda, Regulation of necroptosis signaling and cell death by reactive oxygen species. Biol. Chem. 397, 657–660 (2016)
H.A. Wahba, H.A. El-Hadaad, Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 12, 106–116 (2015)
G.C. Guo, J.X. Wang, M.L. Han, L.P. Zhang, L. Li, microRNA-761 induces aggressive phenotypes in triple-negative breast cancer cells by repressing TRIM29 expression. Cell. Oncol. 40, 157–166 (2017)
E. Robles-Escajeda, U. Das, N.M. Ortega, K. Parra, G. Francia, J.R. Dimmock, A. Varela-Ramirez, R.J. Aguilera, A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell. Oncol. 39, 265–277 (2016)
I. Fkih, M.P. M’hamed, F. Ponelle, F. Penault-Llorca, A. Kenani, Y.-J. Bignon, Identification of miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative breast cancer biomarkers. Cell. Oncol. 38, 433–442 (2015)
T.F. Franke, C.P. Hornik, L. Segev, G.A. Shostak, C. Sugimoto, PI3K/Akt and apoptosis: size matters. Oncogene 22, 8983–8998 (2003)
H. Zhou, X.M. Li, J. Meinkoth, R.N. Pittman, Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 151, 483–494 (2000)
P.D. Ray, B.W. Huang, Y. Tsuji, Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990 (2012)
Y. Son, Y.K. Cheong, N.H. Kim, H.T. Chung, D.G. Kang, H.O. Pae, Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal. Transduct. 2011, 792639 (2011)
S. Cagnol, J.C. Chambard, ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 277, 2–21 (2010)
R.S. Keshari, A. Verma, M.K. Barthwal, M. Dikshit, Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 114, 532–540 (2013)
V. Lobo, A. Patil, A. Phatak, N. Chandra, Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4, 118–126 (2010)
J.F. Turrens, Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335–344 (2003)
A. Valencia, J. Moran, Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic. Biol. Med. 36, 1112–1125 (2004)
F. Ciardiello, R. Caputo, R. Bianco, V. Damiano, G. Pomatico, S. De Placido, A.R. Bianco, G. Tortora, Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 6, 2053–2063 (2000)
M. Villasana, G. Ochoa, S. Aguilar, Modeling and optimization of combined cytostatic and cytotoxic cancer chemotherapy. Artif. Intell. Med. 50, 163–173 (2010)
S.S. Bacus, A.V. Gudkov, M. Lowe, L. Lyass, Y. Yung, A.P. Komarov, K. Keyomarsi, Y. Yarden, R. Seger, Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 20, 147–155 (2001)
Y.H. Choi, Y.H. Yoo, Taxol-induced growth arrest and apoptosis is associated with the upregulation of the Cdk inhibitor, p21WAF1/CIP1, in human breast cancer cells. Oncol. Rep. 28, 2163–2169 (2012)
Acknowledgements
This research was financially supported by Science Foundation Ireland (SFI), grant number 13/IA/1994 (to AMH). Y. Smith was funded by the Health Research Board of Ireland (HRA-POR-2014-545, to AMH). We thank the NCI/DTP Open Chemical Repository (https://dtp.cancer.gov) for the opportunity to work with the natural compound NSC210236, and Dr. Adrienne Gorman for advice on cell death assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
ESM 1
Supplementary Figure 1: C4 alters cellular morphology in MDA-MB-231 cells. MDA-MB-231 cells were plated at either 300,000 or 100,000 per well and treated 48h later with C4 (5μM) or vehicle control (DMSO) for either 24h (A) or 48h (B) for 48h. Cells were then imaged at 40x magnification on an Olympus CKX41 microscope using Cell B imaging software. Supplementary Figure 2: C4 alters nuclear morphology in MDA-MB-231 cells and 4T1 cells. MDA-MB-231 cells were plated at 75,000 per well and treated 48h later and 4T1 cells plated at 50,000 cells per well and treated 24h later with C4 (5μM) or vehicle control (DMSO) for either 24h (A) or 48h (B). Nuclei were then stained with DAPI and imaged at 40x magnification on an Olympus CKX41 microscope using Cell B imaging software. (PPTX 4792 kb)
Rights and permissions
About this article
Cite this article
Richards, C.E., Vellanki, S.H., Smith, Y.E. et al. Diterpenoid natural compound C4 (Crassin) exerts cytostatic effects on triple-negative breast cancer cells via a pathway involving reactive oxygen species. Cell Oncol. 41, 35–46 (2018). https://doi.org/10.1007/s13402-017-0357-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0357-1