Abstract
Purpose
The tumor suppressor protein p53 is known to control cell cycle arrest and apoptosis. Lupeol is a phytochemical that has been found to induce apoptosis in different cancer types through the extrinsic pathway. As yet, however, its role in the induction of cell cycle arrest and apoptosis through the intrinsic pathway in head and neck cancer has not been investigated. Here, we aimed at understanding the mechanism underlying the antitumor effect of Lupeol in head and neck cancer.
Methods
The antitumor effect of Lupeol on oral and laryngeal carcinomas was assessed using two in vitro 2D cell line models (HEp-2, UPCI:SCC-131) and, subsequently, an ex vivo 3D tumor explant culture platform that maintains key features of the native tumor microenvironment. The mechanism underlying Lupeol-mediated antitumor responses was delineated using MTT, colony formation, flow cytometry, immunofluorescence, Western blotting and immunohistochemistry assays.
Results
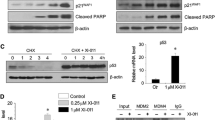
We found that Lupeol induced an enhanced expression of p53 in both cell line models tested and, subsequently, cell cycle arrest at the G1 phase. In addition we found that, following Lupeol treatment, p53 induced Bax expression and activated the intrinsic apoptotic pathway (as measured by Caspase-3 cleavage). Interestingly, Lupeol was also found to trigger G1 cell cycle arrest through up-regulation of the expression of CDKN2A, but not p21, resulting in inhibition of CyclinD1. In an ex vivo platform Lupeol was found to impart a potent antitumor response as defined by inhibition of Ki67 expression, decreased cell viability and concomitant activation (cleavage) of Caspase-3. Finally, we found that Lupeol can re-sensitize primary head and neck squamous cell carcinoma (HNSCC) tumor samples that had clinically progressed under a Cisplatin treatment regimen.
Conclusion
Together, our data indicate that Lupeol may orchestrate a bifurcated regulation of neoplastic growth and apoptosis in head and neck cancers and may serve as a promising agent for the management of tumors that have progressed on a platinum-based treatment regimen.





Similar content being viewed by others
References
Z.A. Stewart, J.A. Pietenpol, p53 signaling and cell cycle checkpoints. Chem Res Toxicol 14, 243–256 (2001)
J.A. Pietenpol, T. Tokino, W.S. El-Deiry, K.W. Kinzler, B. Vogelstein, Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci USA 91, 1998–2002 (1994)
G. Farmer, J. Bargonetti, H. Zhu, P. Freidman, R. Prywes, C. Prives, Wild-type transcription in vivo. Nature 358, 83–85 (1992)
E. Tasdemir, M. Chiara Maiuri, E. Morselli, A. Criollo, M. D’Amelio, M. Djavaheri-Mergny, F. Cecconi, N. Tavernarakis, G. Kroemer, A dual role of p53 in the control of autophagy. Autophagy 4, 1–5 (2008)
A. Rufini, P. Tucci, I. Celardo, G. Melino, Senescence and aging: the critical roles of p53. Oncogene 32, 5129–5143 (2013)
B.T. Spike, G.M. Wahl, p53, stem cells, and reprogramming: tumor suppression beyond guarding the genome. Genes and Cancer 2, 404–409 (2011)
G.P. Zambetti, J. Bargonetti, K. Walker, C. Prives, A.J. Levine, Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev 6, 1143–1152 (1992)
K. Velmeluen, D.R.V. Bockstaele, Z.N. Berneman, The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36, 131–149 (2003)
X.W. Wang, Q. Zhan, J.D. Coursen, M.A. Khan, H.U. Kontny, L. Yu, M.C. Hollander, P.M. O’Connor, A.J. Fornace AJ, C.C. Harris, GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA 96, 3706–3711 (1999)
Z.A. Stewart, S.D. Leach, J.A. Pietenpol, p21Waf1/CiP1 inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol 19, 205–215 (1999)
H. Igaki, H. Sasaki, T. Kishi, H. Sakamoto, Y. Tachimori, H. Kato, H. Watanabe, T. Sugimura, M. Terada, Highly frequent homozygous deletion of the p16 gene in esophageal cancer cell lines. Biochem Biophys Res Commun 203, 1090–1095 (1994)
E. Hara, R. Smith, D. Parry, H. Tahara, S. Stone, G. Peters, Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 16, 859–867 (1996)
M. Gasco, S. Shami, T. Crook, The p53 pathway in breast cancer. Breast Cancer Res 4, 70–76 (2002)
J.P. Morton, P. Timpson, S.A. Karim, R.A. Ridgway, D. Athineos, B. Doyle, N.B. Jamieson, K.A. Oien, A.M. Lowy, V.G. Brunton, M.C. Frame, T.R. Evans, O.J. Sansom, Mutant p53 drives metastasis and overcomes growth arrest/ senescence in pancreatic cancer. Proc Natl Acad Sci USA 107, 246–251 (2010)
M.O. Hengartner, Apoptosis: corralling the corpses. Cell 104, 325–328 (2000)
G. Karp, Cell and molecular biology: concepts and experiments, 5th edn. (Wiley, New Jersey, 2008), pp. 653–657
M. Müller, S. Wilder, D. Bannasch, p53 activates the CD95 (APO-1/Fas) Gene in response to DNA damage by anticancer drugs. J Ex Med 188, 2033–2045 (1998)
M. Bennett, K. Macdonald, S.W. Chan, J.P. Luzio, R. Simari, P. Weissberg, Cell surface trafficking of Fas: a rapid mechanism of p53 mediated apoptosis. Science 282, 290–293 (1998)
J. Yu, Z. Wang, K.W. Kinzler, B. Vogelstein, L. Zhang, PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100, 1931–1936 (2003)
A. Goldman, B. Majumder, A. Dhawan, S. Ravi, D. Goldman, M. Kohandel, P.K. Majumder, S. Sengupta, Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat Commun 6, 1–11 (2015)
A.J. Raffo, H. Perlman, M.W. Chen, M.L. Day, J.S. Streitman, R. Buttyan, Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res 55, 4438–4445 (1995)
S. Fulda, E. Meyer, K.M. Debatin, Inhibition of TRAIL-induced apoptosis by bcl-2 overexpression. Oncogene 21, 2283–2294 (2000)
A.J. Minn, C.M. Rudin, L.H. Boise, C.B. Thompson, Expression of Bcl-XL can confer a multidrug resistance phenotype. Blood 86, 1903–1910 (1995)
F. Vikhanskaya, M.K. Lee, M. Mazzoletti, M. Broggini, K. Sabapathy, Cancer derived p53 mutants suppress p53-target gene expression–potential mechanism for gain of function of mutant p53. Nucleic Acids Res 35, 2093–2104 (2007)
R.B. Lopes, R. Gangeswaran, I.A. McNeish, Y. Wang, N.R. Lemoine, Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer 120, 2344–2352 (2007)
D. Vucic, H.R. Stennicke, M.T. Pisabarro, G.S. Salvesen, V.M. Dixit, MLIAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr Biol 10, 1359–1366 (2000)
Y. Ashhab, A. Alian, A. Polliack, A. Panet, D.B. Yehuda, Two splicing variants of a new inhibitor of apoptosis gene with different biological properties and tissue distribution pattern. FEBS Lett 495, 56–60 (2001)
G. Kaur, S.M. Stetler, S. Sebers, P. Worland, H. Sedlacek, C. Myers, J. Czech, R. Naik, E. Sausville, Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavones. J Natl Cancer Inst 84, 1736–1740 (1992)
A.M. Senderowicz, Development of cyclin-dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia 15, 1–9 (2001)
A.M. Senderowicz, E.A. Sausville, Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst 92, 376–387 (2000)
R. Hoessel, S. Leclerc, J.A. Endicott, M.E. Nobel, A. Lawrie, P. Tunnah, M. Leost, E. Damiens, D. Marie, D. Marko, E. Niederberger, W. Tang, G. Eisenbrand, L. Meijer, Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol 1, 60–67 (1999)
K. Bojanowski, K. Nishio, M. Fukuda, A.K. Larsen, N. Saijo, Effect of suramin on p34cdc2 kinase in vitro and in extracts from human H69 cells: evidence for a double mechanism of action. Biochem Biophys Res Commun 203, 1574–1580 (1994)
L. Meijer, A. Borgne, O. Mulner, J.P. Chong, J.J. Blow, N. Inagaki, M. Inagaki, J.G. Delcros, J.P. Moulinoux, Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 243, 527–536 (1997)
P.J. Moos, K. Edes, J.E. Mullally, F.A. Fitzpatrick, Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis 25, 1611–1617 (2004)
Q.B. She, A.M. Bode, W.Y. Ma, N.Y. Chen, Z. Dong, Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular- signal-regulated protein kinases and p38 kinase. Cancer Res 6, 1604–1610 (2001)
H.S. Choi, M.C. Cho, H.G. Lee, D.Y. Yoon, Indole-3-carbinol induces apoptosis through p53 and activation of caspase-8 pathway in lung cancer A549 cells. Food Chem Toxicol 48, 883–890 (2010)
M.B.C. Gallo and M.J. Sarachine, Biological activities of Lupeol. International journal of biomedical and pharmaceutical sciences. global science books. (2009)
N. Khan, F. Afaq, H. Mukhtar, Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis 28, 233–239 (2007)
M. Saleem, N. Maddodi, M.A. Zaid, N. Khan, B.B. Hafeez, M. Asim, Y. Suh, J.M. Yun, V. Setaluri, H. Mukhtar, Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin Cancer Res 14, 2119–2127 (2008)
B. Majumder, U. Baraneedharan, S. Thiyagarajan, P. Radhakrishnan, H. Narasimhan, M. Dhandapani, N. Brijwani, D. Dency, D.D. Pinto, A. Prasath, U. Basavaraja, B.U. Shanthappa, A. Thayakumar, R. Surendran, G.K. Babu, A.M. Shenoy, M.A. Kuriakose, G. Bergthold, P. Horowitz, M. Loda, R. Beroukhim, S. Agarwal, S. Sengupta, M. Sundaram, A.N.D.P.K. Majumder, Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat Commun 6, 6169 (2015)
B. Abdulkarim, S. Sabri, E. Deutsch, H. Chagraoui, L. Maggiorella, J. Thierry, F. Eschwege, W. Vainchenker, S. Chouaïb, J. Bourhis, Antiviral agent Cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene 21, 2334–2346 (2002)
N. Cidlinsky, G. Dogliotti, T. Pukrop, R. Jung, F. Weber, M.P. Krahn, Inactivation of the LKB1-AMPK signaling pathway does not contribute to salivary gland tumor development - a short report. Cell Oncol 39, 389–396 (2016)
Y. You, W. Yang, X. Qin, F. Wang, H. Li, C. Lin, W. Li, G. Cunguo, Y. Zhang, Y. Ran, ECRG4 acts as a tumor suppressor and as a determinant of chemotherapy resistance in human nasopharyngeal carcinoma. Cell Oncol 38, 205–214 (2015)
M.B. Duz, O.F. Karatas, E. Guzel, N.F. Turgut, M. Yilmaz, C.J. Creighton, M. Ozen, Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study. Cell Oncol 39, 187–193 (2016)
I.P. Ribeiro, F. Caramelo, F. Marques, A. Domingues, M. Mesquita, L. Barroso, H. Prazeres, M.J. Julião, I.P. Baptista, A. Ferreira, J.B. Melo, I.M. Carreira, WT1, MSH6, GATA5 and PAX5 as epigenetic oral squamous cell carcinoma biomarkers - a short report. Cell Oncol 39, 573–582 (2016)
S. Bhattacharyya, S. Mandal, S. Banerjee, G.K. Mandal, A.K. Bhowmick, N. Murmu, Cannabis smoke can be a major risk factor for early-age laryngeal cancer- a molecular signaling-based approach. Tumour Biol 36, 6029–6036 (2015)
D. Královcová, M. Pejchalová, E. Rudolf, M. Cervinka, Quantitative analysis of expression of level of Bcl2 and bax genes in HEp-2 and HL-60 cells after treatment with etoposide. Acta Medica 51, 191–195 (2008)
E.E. Balint, K.H. Vousden, Activation and activities of the p53 tumour suppressor protein. Br J Cancer 85, 1813–1823 (2001)
T.J. Mackey, A. Borkowski, P. Amin, S.C. Jacobs, N. Kyprianou, Bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology 52, 1085–1090 (1998)
Q. Huang, F. Li, X. Liu, W. Li, W. Shi, F.F. Liu, B. O’Sullivan, Z. He, Y. Peng, A. Tan, L. Zhou, J. Shen, G. Han, X.J. Wang, J. Thorburn, A. Thorburn, A. Jimeno, D. Raben, J.S. Bedford, C.Y. Li, Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 17, 860–866 (2011)
D. Cunningham, Y. Humblet, S. Siena, D. Khayat, H. Bleiberg, A. Santoro, D. Bets, M. Mueser, A. Harstrick, C. Verslype, I. Chau, E.V. Cutsem, Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351, 337–345 (2004)
Z. Hosseini, A. Ghorbani, Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed 5, 84–97 (2015)
S. Rauth, S. Ray, S. Bhattacharyya, D.G. Mehrotra, N. Alam, G. Mondal, P. Nath, A. Roy, J. Biswas, N. Murmu, Lupeol evokes anticancer effects in oral squamous cell carcinoma by inhibiting oncogenic EGFR pathway. Mol Cell Biochem 417, 97–110 (2016)
M.F. Roussel, The INK4 family of cell cycle inhibitors in cancer. Oncogene 20, 5311–5317 (1999)
H. Gali-Muhtasib, R. Hmadi, M. Kareh, R. Tohme, N. Darwiche, Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis 20, 1531–1562 (2015)
S.S. Lin, H.P. Huang, J.S. Yang, J.Y. Wu, T.C. Hsia, C.C. Lin, C.W. Lin, C.L. Kuo, W.G. Wood, J.G. Chung, DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett 272, 77–90 (2008)
G. Ghavami, S. Sardari, M.A. Shokrgozar, Cheminformatics-based selection and synergism of herbal extracts with anticancer agents on drug resistance tumor cells—ACHN and A2780/CP cell lines. Comput Biol Med 41, 665–674 (2011)
C.H. Park, E.R. Hahm, S. Park, H.K. Kim, C.H. Yang, The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett 579, 2965–2971 (2005)
D. Fong, A. Yeh, R. Naftalovich, T.H. Choi, N.M. Chan, Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett 293, 65–72 (2010)
K. Sak, Chemotherapy and dietary phytochemical agents. Chemother Res Pract 2012, 1–11 (2012)
Acknowledgements
The authors acknowledge the Department of Science and Technology (DST), Government of India, for funding this project. We are also grateful to Dr. Anthony Gomes, Department of Physiology, Calcutta University, for allowing us to use the FACSVerse™ platform and to Biplab Tewary for IHC assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest for all authors
None declared.
Electronic supplementary material
ESM 1
(DOCX 11 kb)
Rights and permissions
About this article
Cite this article
Bhattacharyya, S., Sekar, V., Majumder, B. et al. CDKN2A-p53 mediated antitumor effect of Lupeol in head and neck cancer. Cell Oncol. 40, 145–155 (2017). https://doi.org/10.1007/s13402-016-0311-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-016-0311-7



