Abstract
Purpose
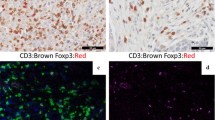
T regulatory cells, a subset of T lymphocytes, function to suppress immune responses. FOXP3, a member of the forkhead family of transcription factors, is a good marker for T regulatory cells. Since sentinel nodes are important sites of immunomodulation in breast cancer, we studied the association between T regulatory cells and nodal metastasis using FOXP3 expression in sentinel nodes with and without metastatic breast carcinoma.
Methods
Following sample size calculations, we selected 140 sentinel nodes from breast cancer patients; 70 with metastasis (sentinel node+) and 70 without metastasis (sentinel node–). FOXP3 expression in sentinel nodes was studied by immunohistochemistry. Cortical cells expressing FOXP3 were counted in 10 high power fields.
Results
In the evaluable cases, the node positive (n = 66) and negative (n = 69) groups were well balanced for all clinicopathological parameters except histological type. The mean number of T regulatory cells expressing FOXP3 (per 10 hpf) was 139 in the node positive and 132 in the node negative group (P = 0.540). The mean number of T regulatory cells was 162 in patients ≤35 years of age (n = 8) compared to 133 in older patients (P = 0.250). Primary tumor pathological characteristics like tumor type, grade, size, and ER, PR, and HER2 status did not correlate with FOXP3 expression.
Conclusions
The number of FOXP3-expressing T regulatory cells does not differ significantly between sentinel node+ and sentinel node– samples. It was also not affected by primary tumor characteristics like tumor type, grade, size, hormone receptor, and HER2 status.

Similar content being viewed by others
References
T.R. Mosmann, R.L. Coffman, TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989)
J.I. Ellyard, L. Simson, C.R. Parish, Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens 70, 1–11 (2007)
H. Murakami, H. Ogawara, H. Hiroshi, Th1/Th2 cells in patients with multiple myeloma. Hematology 9, 41–45 (2004)
A.M. Thornton, E.M. Shevach, CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998)
R.M. Lopez, M. Moser, Dendritic cell subsets and the regulation of Th1/Th2 responses. Sem. Immunol. 13, 275–282 (2001)
R.B. Effros, Replicative senescence of CD8 T cells: potential effects on cancer immune surveillance and immunotherapy. Cancer Immunol. Immunother. 53, 925–933 (2004)
Z. Fehervari, S. Sakaguchi, CD4+ Tregs and immune control. J. Clin. Invest. 114, 1209–1217 (2004)
M. Dougan, G. Dranoff, Immune therapy for cancer. Annu. Rev. Immunol. 27, 83–117 (2009)
S. Hori, T. Nomura, S. Sakaguchi, Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003)
B.K. Halak, H.C. Maguire Jr., E.C. Lattime, Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 59, 911–917 (1999)
F. Ichihara, K. Kono, A. Takahashi, H. Kawaida, H. Sugai, H. Fujii, Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. 9, 4404–4408 (2003)
C. Rieser, R. Ramoner, L. Holtl, H. Rogatsch, C. Papesh, A. Stenzl, G. Bartsch, M. Thurnher, Mature dendritic cells induce T-helper type-1-dominant immune responses in patients with metastatic renal cell carcinoma. Urol. Int. 63, 151–159 (1999)
J.D. Fontenot, M.A. Gavin, A.Y. Rudensky, Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003)
S. Sakaguchi, The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J. Clin. Invest. 112, 1310–1312 (2003)
M. Gobert, I. Treilleux, N. Bendriss-Vermare, T. Bachelot, S. Goddard-Leon, V. Arfi, C. Biota, A.C. Doffin, I. Durand, D. Olive, S. Perez, N. Pasqual, C. Faure, I. Ray-Coquard, A. Puisieux, C. Caux, J.-Y. Blay, C. Ménétrier-Caux, Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 69, 2000–2009 (2009)
A. Merlo, P. Casalini, M.L. Carcangiu, C. Malventano, T. Triulzi, S. Mènard, E. Tagliabue, A. Balsari, FOXP3 expression and overall survival in breast cancer. J. Clin. Oncol. 27, 1746–1752 (2009)
M. Ohara, Y. Yamaguchi, K. Matsuura, S. Murakami, K. Arihiro, M. Okada, Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol. Immunother. 58, 441–447 (2009)
E.G. Mansour, P.M. Ravdin, L. Dressler, Prognostic factors in early breast carcinoma. Cancer 74, 381–400 (1994)
A.E. Giuliano, D.M. Kirgan, J.M. Guenther, D.L. Morton, Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann. Surg. 220, 391–398 (1994). discussion 398–401
D.N. Krag, D.L. Weaver, J.C. Alex, J.T. Fairbank, Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg. Oncol. 2, 335–339 (1993). discussion 340
D.L. Morton, D.R. Wen, J.H. Wong, J.S. Economou, L.A. Cagle, F.K. Storm, L.J. Foshag, A.J. Cochran, Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 127, 392–399 (1992)
N.J. Poindexter, A. Sahin, K.K. Hunt, E.A. Grimm, Analysis of dendritic cells in tumor-free and tumor-containing sentinel lymph nodes from patients with breast cancer. Breast Cancer Res. 6, R408–R415 (2004)
R.R. Huang, D.-R. Wen, J. Guo, A.E. Giuliano, M. Nguyen, R. Offodile, S. Stern, R. Turner, A.J. Cochran, Selective modulation of paracortical dendritic cells and T-lymphocytes in breast cancer sentinel lymph nodes. Breast J. 6, 225–232 (2000)
S. Ishigami, S. Natsugoe, Y. Uenosono, Y. Hata, A. Nakajo, F. Miyazono, M. Matsumoto, S. Hokita, T. Aikou, Infiltration of antitumor immunocytes into the sentinel node in gastric cancer. J. Gastrointest. Surg. 7, 735–739 (2003)
J. Schule, L. Bergkvist, L. Hakansson, B. Gustafsson, A. Hakansson, Down-regulation of the CD3-zeta chain in sentinel node biopsies from breast cancer patients. Breast Cancer Res. Treat. 74, 33–40 (2002)
K. Matsuura, Y. Yamaguchi, H. Ueno, A. Osaki, K. Arihiro, T. Toge, Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 106, 1227–1236 (2006)
A.S. Mansfield, P.S. Heikkila, A.T. Vaara, K.A. von Smitten, J.M. Vakkila, M.H. Leidenius, Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer 9, 231 (2009)
K. Matsuura, Y. Yamaguchi, A. Osaki, M. Ohara, R. Okita, A. Emi, S. Murakami, K. Arihiro, FOXP3 expression of micrometastasis-positive sentinel nodes in breast cancer patients. Oncol. Rep. 22, 1181–1187 (2009)
R. Nakamura, M. Sakakibara, T. Nagashima, T. Sangai, M. Arai, T. Fujimori, S. Takano, T. Shida, Y. Nakatani, M. Miyazaki et al., Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur. J. Cancer 45, 2123–2131 (2009)
S.P.L. Leong, M. Peng, Y.M. Zhou, J.E. Vaquerano, J.W.C. Chang, Cytokine profiles of sentinel lymph nodes draining the primary melanoma. Ann. Surg. Oncol. 9, 82–87 (2002)
Acknowledgements
SB is supported by Susan B Komen Scholar Grant.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gökmen-Polar, Y., Thorat, M.A., Sojitra, P. et al. FOXP3 expression and nodal metastasis of breast cancer. Cell Oncol. 36, 405–409 (2013). https://doi.org/10.1007/s13402-013-0147-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-013-0147-3