Abstract
Blue-green algae Arthrospira platensis, strain NIOF 17/003, is an ecologically friendly were tested as adsorbents for the removal of methyl orange dye (MO) from aqueous solution. The surface deposition of A. platensis was investigated utilizing a variety of techniques, including Fourier-transform infrared spectroscopy (FT-IR), Brunauer–Emmett–Teller (BET), and scanning electron microscopy (SEM). FTIR analysis demonstrated the contribution of carbonyl and carboxylic groups to MO dye adsorption. Different initial concentrations of MO dye (5–30 mg L–1) at contact time (5–180 min.), adsorbent doses (0.05–0.4 g), temperature (20–60 °C), and pH (1–11) were conducted in batch conditions. The results revealed that MO sorption in aqueous solutions increased with contact time and concentration. Temperature also affects MO adsorption. The elimination of MO from the solution augmented as the mass of A. platensis increased. The maximum MO dye sorption onto A. platensis was achieved at 15 mg L−1, 20 °C, 0.4 g, pH 1, for 30 min. A significant quantity of dye can be removed with just 0.1 g of A. platensis, with a removal efficiency of 18.1% and 28.35% for seawater and real wastewater, respectively. Many isotherm equations were used to analyze the experimental results (including Freundlich, Langmuir, Dubinin-Radushkevich, and Temkin models). The maximum adsorption capacity of MO onto A. platensis was 156.25 mg g−1. The fractionary-order kinetic model provided the best fit to experimental data compared with other models. Moreover, the thermodynamic parameters of ΔH° and ΔG° factors showed exothermic as well as spontaneous sorption processes. Additionally, A. platensis can be used more than once for economic gain, and it was found that the percent removal decreased to 89.28% of regeneration after three cycles for synthetic wastewater. These investigations indicate the effectiveness of the blue-green algae A. platensis, which may have significant potential for methyl orange dye applications in wastewater treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water is usually essential for human survival as well as the survival of almost all ecosystems [1]. Water contamination caused by technological advancement is still a major concern. Dyes are chemical substances that may connect to surfaces or textiles and produce color. Dyes and pigments are widely used in textiles, plastics, food, paper, leather, colorants in the cosmetic sector, and natural and synthetic fibers [2]. Several industries have been identified as the source of the increasing emission of colored effluents containing synthetic organic dyes [3]. Methyl orange (MO) is one of the most commonly dye used in the textile industry [4, 5]. It is also used as a pH indicator for titrations [6,7,8]. The release of colored wastewater into the environment has negative impacts on the receiving water bodies and poses significant risks since they contaminate natural habitats and are not biodegradable [9]
Aquatic organisms have also been shown to be mutagenic and carcinogenic to synthetic dyes as well as human life, causing major heart, kidney, liver, reproductive, and nervous system malfunctions. Because of their obstinacy, their emissions into the hydrosphere contribute significantly to pollution. This will give the water reducing sunlight penetration, an unattractive color [10].
Over 10,000 tonnes of dye are used worldwide in the textile industry each year, with approximately 100 tonnes of dye discharged into waterways each year [11, 12]. Fundamentally, the exact amount of dyes discharged into the environment from different procedures is unknown. However, environmental scientists have serious worries about the leakage of significant volumes of synthetic dyes into the environment. Some of the procedures used to remove colors from wastewater include membrane separation [13], coagulation, adsorption, and advanced oxidation [14, 15]. However, the preivous menthioned methodology have several disadvantages like the complexity, high operational cost and time-consuming unit operations as stated above [16].
Adsorption is a very successful advanced wastewater treatment process used to diminish dangerous inorganic/organic contaminants in effluent [17, 18]. The goal of the present work is to identify a low-cost alternative to blue-green algae as an adsorbent. Numerous studies have been done to determine whether natural resources, biosorbents, industrial solid wastes, and agricultural byproducts can be used as precursors for different low-cost adsorbents. The study also said that raw biomass adsorbents were subjected to various chemical or physical treatments in order to improve their adsorption ability [19].
Green nanotechnology is a method of nanomaterials production that is free of harmful components or uses less hazardous components throughout the manufacturing process [20]. Biological elements like actinomycetes, bacteria, cyanobacteria, fungi, macroalgae, and plants can all be employed to green synthesize NPs. Green synthesis is chosen over chemical and physical methods because of its affordability, environmental friendliness, scale, simplicity, and biocompatibility [21, 22]. Blue-green algae Arthrospira platensis is a widely grown species with a high yield of 2000 tonnes per year [23]. Arthrospira platensis, a filamentous cyanobacterium with various advantages such as high biomass output, fast growth rates, biomass composition adjustment, and simplicity of cell harvesting, is a possible biosorbent. Arthrospira platensis is a well-known source of polysaccharides, protein, minerals, lipids, phosphate, and vitamins [24], they are all essential for dye-binding [25].
Using a batch method, the effects of numerous parameters such as initial MO concentration, contact time, pH, temperature, and adsorbent dose on the adsorption rate and processes of MO onto A. platensis were examined. In addition, Brunauer–Emmett–Teller (BET), Fourier transform infrared (FT-IR), and scanning electron microscopy (SEM) were used to assess the adsorbent's morphology and physicochemical characteristics. Subsequently, we further analyzed the adsorption capacities and efficiencies, and data of equilibrium used to determine adsorption isotherms, kinetics, and thermodynamics in order to assess the adsorption mechanism and rate constants. This study provides valuable insights for further exploring the potential of blue-green algae, A. platensis, frameworks in dye adsorption applications especially in case of ease of regeneration, cost effective, stability, and quick synthesis. This study has the potential to provide deeper understanding of MO adsorption, thereby enhancing the knowledge base for future work. Additionally, it can minimize the subjective selection of adsorbents for MO adsorption, resulting in a decrease in the volume of published literature that adds little or only marginal value to the field.
2 Materials and methods
2.1 Chemical reagents
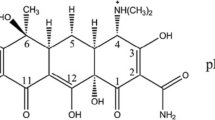
Alpha Chemika in India provided methyl orange (2-(N, N-dimethyl-4-aminophenyl) azo benzene carboxylic acid), which was utilized as a commercial dye. Through diazotization of anthranilic acid, methyl orange had been equipped, after which a reaction with dimethyl aniline occurs. Table 1 shows the chemical formula, molar mass, melting point, kinds of dyes, density, extreme adsorption wavelengths, and molecular structure. To avoid direct light exposure, the equipped stock was kept in the dark. However, 1 g of the dye powder was dissolved in 1 L of distilled water (DW) to make a stock solution. Each adsorption experiment solution was prepared by diluting the stock to the required concentration. The pH of the solution was adjusted using either 1 M HCl or 1 M NaOH.
2.2 Adsorbent preparation
The preparation of A. platensis nanoparticle was caryed out at the Egyptian Petroleum Research Institute (EPRI), Nasr City, Cairo, Egypt, via ball grinding (Planetary Ball Mill PM 400 “4 grinding stations”), as detailed in our previous publication [26]. As demonstrated in our earlier works [26,27,28], the size distribution of A. platensis nanoparticles was determined using dynamic light scattering (DLS), resulting in average sizes of 183.9 nm (87.6%) and 1069 nm (12.4%). In contrast, the typical size of the A. platensis alga was 100 µm [26,27,28].
2.3 Batch adsorption experiments
Experimentations for MO removal were accompanied in an air-tight Erlenmeyer flask (500 mL) by captivating 200 mL of 50 mg L−1 dye solution. The temperature of the system was saved constant at 25 ± 2 °C. Samples were withdrawn and filtered during contact times to isolate the adsorbent from the dye solution. Spectrometric analysis was used to assess the concentration of the dye solution, all experimentations were accomplished. Experiments were carried out to explore the effects of initial dye concentration on dye absorption, with dye concentrations ranging from 5 to 50 mg L−1 at constant dosages of 0.1 g at pH 7 [24, 29].
By changing the initial pH of the dye from 1 to 11, the effects of the final pH on MO adsorption were investigated. Prior to measuring the final pH, the adsorbent and the dye mixture were permitted to equilibrate for 120 min in a shaker at 25 ± 2 °C to the final pH measurement. While the effects of contact time at a range from 10 to 120 min and adsorbent doses (0.05 to 0.4 g) were studied at a constant initial dye concentration of 5 mg L−1 at a constant temperature of 25 ± 2 °C and pH 7. Samples were shaken in a temperature-controlled incubator shaker at 25 °C and 180 rpm. At a predetermined period ranging from 5 to 120 min, samples were withdrawn for spectrophotometric analysis [29, 30].
2.4 Equilibrium studies
To assess the type of adsorption isotherms and the sorption capacity of the adsorbent for MO dye removal, adsorption equilibrium analyses were performed. The processes of adsorption were examined using pseudo-first-order and pseudo-second-order models, as well as an intra-particle diffusion mechanism. The kinetic characteristics were examined for contact times between 10 and 120 min. The parameters of adsorption isotherm models were also considered to know adsorption behavior. The initial dye concentrations for the isotherm studies were varied from 5 to 50 mg l−1 using 0.1 g of biomass of adsorbent. Besides, the thermodynamic investigations were carried out in an incubator shaker for 2 h at different temperatures ranging from 25 to 65 °C in an incubator shaker for 2 h. At the end of the experiments, the samples were filtered, and the residual concentration was calculated. The % elimination of the dye was estimated using the reduction in absorbance at the dye’s maximum value as follows [16]:
where: Ci and Cf (mg L−1) are the initial and final MO concentrations at a given time, respectively; V is the dye mixture volume (L), and W is the dry adsorbent weight (g).
2.5 Characterization of adsorbent material
2.5.1 Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy was used to analyze the chemical structure by VERTEX 70 FT-IR-Bruker spectrometer. The substance was mixed with 1% KBr and crushed into pellets before being studied in the mid-infrared range (500–4000 cm−1) at 4 cm−1 resolution [31].
2.5.2 Scanning electron microscopy (SEM)
Under a 30 kV accelerating voltage, the samples’ surface morphology was examined using a scanning electron microscope (SEM, FEI, Quanta 200, Thermo Scientific, Waltham, MA, USA). SEM was used to verify the morphological variations between the nanoparticles of A. platensis biomass before and after MO dye sorption.
2.5.3 BET investigation
The specific surface area and pore volumes of the adsorbent were measured to assess whether the porosity of the sample under consideration had changed. The nitrogen adsorption–desorption isotherms of impregnated nanoparticles A. platensis at 77.33 K. The textural properties of nano A. platensis were obtained using the instrument (NLDFT Ads. model) analytical software and certain parameters were obtained using BET, t-plot, and BJH-Des techniques.
2.6 Equilibrium isotherm
2.6.1 Langmuir and Freundlich models
The Langmuir model, which is based on the notion that intermolecular interactions drop rapidly with distance and may thus predict the presence of monolayer coverage for the sorbate on the sorbent’s exterior surface, was carefully chosen.
While the Freundlich model is used to demonstrate system heterogeneity and define the reversible sorption process, it does not reveal whether dye ion retention happens in several layers. The linear forms described below were used to compute the mathematical formulas of the Langmuir model (see (3)) and the Freundlich model (see (5)) [32] and [33]:
where: qe denotes the equilibrium adsorption capacity, qmax means the maximum sorption capacity and b represents the Langmuir constant. While, Kf indicates the Freundlich constant, and n represents the heterogeneity factor.
2.6.2 Temkin isotherm model
As indicated by the following linear equation, the Temkin equation posits that the heat of the sorption process reduces linearly with adsorption coverage due to adsorbent-adsorbate interactions [34]:
where: B = (RT b−1) (J mol−1) is the Temkin constant and related to the heat of adsorption, T denotes the absolute temperature (K), R (8.314 J mol−1 K) means the ideal gas constant, and A refers to the value for the higher binding energy’s associated equilibrium binding constant (L min−1).
2.6.3 Dubinin-Radushkevich (D-R) isotherm
Dubinin-Radushkevich’s (D-R) isotherm model was used to assess whether the sorption method was chemical or physical [35]. The Dubinin-Radushkevich model is frequently used in the following equation:
where: The polanyi potential and the adsorption capacity constant, qm (mg g−1), are both present. The adsorption means free energy constant is K (mol2 kJ−2), the sorption energy constant is E (kJ mol−1), the gas constant is R (J mol−1 K−1), and the absolute temperature is T (K).
3 Results and discussion
3.1 Adsorbents characterization
3.1.1 FTIR spectrum
The kind and quantity of functional groups on the adsorbent’s surface significantly affect MO adsorption [36]. Figure 1 depicts the results of the identification of the functional groups of the adsorbent using an FTIR spectrometer. The broadband at the range of 3421 to 3364 cm–1 can be attributable to bond –OH and –NH groups. The peak at 2924 cm–1 indicated the aliphatic C–H groups. The peak at 2855 cm–1 after remediation is caused by asymmetric CH3 stretching and both asymmetric and symmetric CH2 stretching [37], which are commonly observed in microalgal biomass. The band at 2076 cm−1 represents C≡C groups. While the C = O group may be responsible for the peaks around 1637 and 1650 cm–1 before and after remediation. The band created after adsorption at 1545 cm−1 can be assigned to C = C stretch aromatic or N–H bending types of bonds of amide (II). To aromatic rings, this group can be conjugated or unconjugated [38, 39]. While, the absorption band at 1419 cm−1 split into two peaks at 1451, and 1431 cm−1 indicating the presence of sp3 C-H bend. The peak at 1361 cm–1 identified the aliphatic C–H groups. In the A. platensis nanoparticles alga, a deformation related to the C–O bond was observed around 1100–1350 cm−1. Moreover, before and after adsorption, stretching vibrations –C–O was discovered at adsorption peaks at 1048 and 1033 cm–1 [40]. The adsorption bands in the 750–900 cm─1 range are caused by S–O, P-O, and aromatic C-H stretching vibrations [41]. The broadening and shifting of peaks on spectra suggested the interaction of functional groups on the outer layer of the A. platensis cell wall with MO dye ions in an aqueous solution. According to the FTIR spectrum of the adsorbent, MO ions may bind to anionic groups and amino groups by electrostatic attraction. The NH2, C = O and OH groups, in addition to the aromatic groups present in the bio-mass, and the sulfate and phosphate peaks regions, were detected when the adsorbent was loaded with MO.
3.1.2 Scanning electronic microscopy (SEM)
The form, size, and surface features such as morphology were investigated using SEM. A. platensis nanoparticles’ surface shape before and after MO adsorption was depicted in Fig. 2a and b. The SEM morphology of nanoparticles A. platensis was found to be irregularly dispersed and spherical. This showed that the surfaces of microalgal cells were heterogeneous. The active sites were homogeneous before adsorption, which led to the creation of spherical particles with a uniform distribution, according to SEM examination. And the outer layer of cells was found to be uneven, porous, with aggregation, irregularity, and small particles covering it after MO adsorption. The SEM image of the cells following sorption also reveals that amorphous materials gathered all over the cell surface. It was possible to see pores and tubes of different sizes and forms. It is believed that the small particles contain released cell contents from the therapy. According to Adeyi, et al. [42], the MO cations created a MO ion monolayer on the surface of the A. platensis nanoparticles by covering the adsorbent particles and filling in the gaps.
3.1.3 BET examination
The application of the adsorption method is most strongly influenced by the physical characteristics of the adsorbent as assessed by BET analysis. The specific surface area of A. platensis nanoparticles was examined using the N2 sorption isotherms at 77 K. Figure 3 displays the nitrogen adsorption/desorption isotherms. The surface area of the A. platensis nanoparticles measured by multipoint BET and single point BET, respectively, was 139.83 m2 g−1 and 114.373 m2 g−1. For the synthetic adsorbent, this is a reasonable value. It is important to note that MO dye is absorbed from an aqueous solution more effectively the larger the surface area. The BJH adsorption cumulative micropore volume was 0.101 cc g─1. While the BJH adsorption pore radius was 1.55779 nm, however, the BJH desorption pore radius was 1.63 nm. Since algal swelling in aqueous solutions increases the surface area of A. platensis nanoparticles, the results demonstrate the particles had a significant surface area.
3.2 Factors influencing batch adsorption
3.2.1 Influence of contact time and initial MO concentration
The initial concentration effect (5, 10, 20, 30 mgL−1) on MO adsorption onto 0.1 g of A. platensis at various times (5, 10, 15, 30, 60, 120, and 180 min) and a constant rate of 150 min was studied. The findings are examined, and the results are summarized in Fig. 4. An increase in the adsorption process was observed from 3.7 to 17 mg g−1 for raw biomass with a 5 mgL−1 initial concentration of MO. Figure 4 shows that for MO concentrations of 5, 10, 20, and 30 mg L−1, the removal % was 70, 25.4, 52.3, and 51.6, respectively, for the initial MO concentration of 0.1 g at 180 min. This findings demonstrated that the sorption capacity increases when the concentration of the initial MO dye increases. This suggests that the initial dye concentration has an impact on dye sorption. Ion exchange or physical adsorption at the cell surface may be involved in the initial rapid phase, while other mechanisms such as binding site saturation, micro-precipitation, or complexation may be included in the subsequent slower phase. It is possible that this is a result of the driving force to break through all dye molecule mass transfer barriers among the solid and aqueous phases [43].
The initial concentration of MO dye ions (which could conflict with the ions of interest for active sorption sites) also influences the adsorption rate [44]. At the initial stage, the adsorbent’s surface is bare; sorption occurs rapidly and is normally controlled by a diffusion process from the bulk to the surface. Adsorption is likely an attachment-controlled process at this stage since there are fewer potential sorption sites [44].
The equilibration time necessary for sorption was found to be around 30 min for all initial MO dye concentrations because the equilibrium reaching time was independent of dye concentration, demonstrating that rapid adsorption happened and that sorption was not governed by film diffusion from bulk to surface [43]. The typical route taken by the dye molecules from the bulk solution to the exterior surface of the adsorbent via the liquid film, and then from the exterior surface of the adsorbent to the pores, demonstrates that the initial concentration of dye is significantly increased by the dye adsorption onto the adsorbent [43]. For a given adsorbent dose, the total number of potential adsorption sites is typically fixed. It is reasonable to assume that at lower initial concentrations, the adsorbent has a larger ratio of active adsorption sites. As a result, at lower initial concentrations, the percentage of dye removed is greater, whereas at higher initial concentrations, it is lower.
3.2.2 Effect of adsorbent dosage
The influence of adsorbent dose provides a clue as to a dye’s ability to sorb with the least quantity of adsorbent possible, enabling the economical recognition of a dye’s potential. The adsorbent dose is also a significant restriction on dye adsorption, which was also studied for biomass dosage in values 0.05, 0.1, 0.2, 0.3, and 0.4 g 50 mL−1 range while other factors remain unchanged. Figure 5 summarizes the effect of adsorbent dosage. The MO amount eliminated is greatly dependent on the adsorbent dose. The maximum dye adsorption of 40.7, 46.1, 47.1, 47.2, and 47.7 (mg g−1) in biomass dosages of 0.05, 0.1, 0.2, 0.3, and 0.4 g, respectively. Besides, the percentage of dye removal increases with increasing biomass dosage from 0.05 to 0.4 g with percentage (92.6 to 95.6%). By a fixed initial dye concentration, an increase in increase in the adsorbent surface area and availability of more adsorbent sites resulted in an increase in percentage removal % and MO adsorption capacity [45, 46]. Furthermore, this is most likely due to dye mass transfer resistance from bulk liquid to a solid surface, which becomes significant at great adsorbent loading, as was shown in the experiment [47, 48].
Nevertheless, increasing the adsorbent dosage leads to little improvement in the adsorbent. This could be because adsorbent particles collide more frequently, as a result, there are fewer unoccupied sites available for adsorption per unit mass of adsorbent, and such collisions may result in active site aggregation or overlapping. As a result, 0.04 g was selected as the optimized adsorbent dose and was used for the rest of the experiment [49]. In general, when the amount of sorption sites rises at the surface of the adsorbent with increasing adsorbent dose, therefore, the dye removal increases [50].
3.2.3 Influence of solution pH
The sorption process depends on the dye solution’s pH, particularly its adsorption capacity. As shown in Fig. 6, the effect of different solution pH (1–11) on the equilibrium uptake of MO dye was studied at constant levels of other variables. The dye uptake was shown to decrease as the pH increased. Various species adsorbed on different adsorbents will have varied pH ranges for adsorption. When 87.52% of the MO was removed, the adsorbent’s maximum adsorption capacity was 25.719 mg g−1 at pH 1 at an initial dye concentration of 5 mgL−1 at 25 °C. The amount of dye removal decreased by 39% in the pH range of 1 to 7. Founded on current data, the initial pH of all adsorbate solutions was kept at 1. While, the adsorbent’s major sorption capacity for MO adsorption occurs around pH 1.0–3.0 because the amino groups are protonated at this pH range, facilitating the adsorption of the negatively charged dye [51]. This also suggested that the electronic interaction between adsorbent and adsorbate was not the dominant factor. Furthermore, the dimer form may be more difficult to permeate through the micropores of the adsorbent particle than the smaller monomeric MO [49].
3.2.4 Influence of temperature
At an initial concentration of 5 mg l−1, the impact of temperature on the equilibrium alga sorption capacity for MO was examined over the temperature range of 20–60 °C. As presented in Fig. 7, the equilibrium uptake of MO reduced with boosting temperature demonstrating that sorption between adsorbent and MO was an exothermic process with physical adsorption being the main mechanism at lower temperatures. At 20 °C, the maximum equilibrium absorption of MO was 99.83%, with an equilibrium sorption capacity of 49.78 mg g−1. Adsorption of MO was likewise oppositely proportional to temperature increase, i.e. increasing temperature until 60 °C resulted in a reduction in equilibrium uptakes from 98.73% (20 °C) to 96.78% (60 °C) due to decreased surface activity. Also, a reduction in temperature increases adsorption, a phenomenon that is observed in physical adsorption [52].
3.3 Thermodynamic study
In to decide which process will occur spontaneously, every adsorption process needs to take into account entropy factors. The actual indicators for a process’s practical applicability are the values of thermodynamic parameters. The amount of MO adsorbed onto the adsorbent at equilibrium at various temperatures (20–60 °C) was examined (Table 2) in order to determine thermodynamic parameters for the adsorption system. The thermodynamic parameters such as enthalpy (ΔHo), a change in the standard free energy (ΔGo), and entropy (ΔSo) were calculated by applying the following equations:
The value uses of enthalpy and entropy was calculated from the intercept and slope of the plotted curve of temperature vs. change in the standard free energy of Eq. (11) or (12), as well as ∆G°, which can be calculated from Eq. (13). The values of thermodynamic factors for the adsorption process are presented in Table 2.
The estimated negative value of (ΔH° = − 70.88 kJ mol−1) showed that the adsorption process was exothermic; however, the positive values of entropy change (ΔS° = 0.1933 J mol−1 K−1) confirmed the high preference for MO molecules on the adsorbent surface [53]. The low negative enthalpy change values indicate exothermic and physical adsorption [54]. The values of ∆G° decreased from − 6.95 to − 4.63 kJ mol−1 for the Langmuir isotherm and ascended from − 13.99 to − 7.52 kJ mol−1 as the temperature increased from 20 to 60 °C. The minus sign of the change in free energy (∆G°) confirms the feasibility of adsorption processes and also reveals spontaneous adsorption of MO onto adsorbent in the temperature range studied. The average ∆G° value for the MO adsorption developed in this work is − 10.1 kJ mol−1, indicating that the dominating mechanism was physical adsorption via a physical process involving weak attraction forces. At the same time, the low value of ΔHo suggests that the adsorbate molecules were loosely bound to the adsorbent surface [55].
3.4 Adsorption isotherms
An adsorption isotherm describes the relationship between the concentration of adsorbate still present in the solution and the amount of adsorbate absorbed by the adsorbent. These equilibrium models’ equation variables often show the adsorbent’s affinity, surface properties, and adsorption mechanism. In this analysis, four significant isotherm models were chosen to account for the experimental equilibrium results.
3.4.1 Langmuir
According to the Langmuir equation, an adsorbed solute monolayer will form [56]. The monomolecular sorption capacity (qm), comparable to full monolayer coverage on the biomass surface, was calculated using the Langmuir adsorption isotherm. Table 3 shows the values of qm, b, and the coefficient, R2. The outcomes show that the Langmuir equation is followed during the sorption of MO dye ions onto an adsorbent, as seen through the high correlation values R2 = 0.999 as presented in Fig. 8. The monomolecular adsorption capacity qmax showed that the adsorbent (156.25 mg g−1) has a higher mass capacity. The separation factor was used to investigate the interaction of dye ions with adsorbents further (RL). The equilibrium parameter RL of the Langmuir model is a dimensionless constant separation factor. The RL was defined by Hall et al. [57] and is expressed as Eq. (16).
where: Ci is the initial concentration of dye ion (mg L−1), while b is the Langmuir constant (L mg−1). As defined by Hall et al. [57], the RL values indicate whether the isotherm is favorable (0 < RL < 1), linear (RL = 1), unfavorable (RL > 1), or irreversible (RL = 0). Therefore, the RL values for MO adsorption onto adsorbents are in the range of 0–1 with an average value of 0.05. As stated in Table 3, the elimination of MO ions was beneficial.
3.4.2 Freundlich
The adsorption equilibrium of MO dye on active and physically processed algal biomass preparations was predicted using the Freundlich data. The Freundlich isotherm equation was used to fit the experimental data, as shown in Fig. 9, and it had a strong correlation (R2 = 0.988). On the basis of adsorption onto a heterogeneous surface, the Freundlich equation was developed [58]. For MO dye onto adsorbent, KF and n parameters were discovered to be 159.2 and 1.12, respectively (Table 3). For favorable adsorption, the Freundlich constant n should be in the range of 1 to 10. Positive binding and heterogeneous adsorption are indicated by n > 1 values [27].
3.4.3 Temkin
The Temkin isotherm equation predicts that binding energies will be distributed uniformly throughout the population of exterior binding sorption sites. Furthermore, the range and distribution of binding energies should be significantly influenced by the density and distribution of functional groups across both the dye and biosorbent surfaces. The Temkin adsorption isotherm model was selected to determine the adsorption potentials of the adsorbent for adsorbates. Table 1 shows the isotherm parameters. The R2 values obtained for the linear models were close to unity (> 0.759) for the entire set of isotherm models investigated as shown in Fig. 10. As a result of this study, isotherm models appear to be less effective in modeling experimental data. Alternatively, the Temkin isotherm’s bT constant is connected to adsorption energy variation. A positive value of the bT constant suggests an exothermic adsorption reaction, as demonstrated by the value of 24.5 in this study [59].
3.4.4 Dubinin-Radushkevich
Dubinin-Radushkevich (D-R) isotherm is another isotherm equation that assumed that the sorption curve characteristics are related to the adsorbent’s porosity. Table 3 shows the isotherm parameters qm and K. The linear correlation coefficient is R2 = 0.86 as exhibited in Fig. 11. The average sorption energy E (kJ mol−1) was found to be 10, which is in the energy range of physical sorption reactions. The biomass has a monomolecular adsorption capacity qm of 24.78 mol g−1 [60].
3.4.5 Goodness of model fit
Traditional approaches for estimating isotherm variables, such as linear regression equations, appear to provide very good fits to experimental results. To evaluate the fit of isotherm equations to experimental data, a large number of linear regression basin error functions were used to estimate isotherm constants and compare them to less precise linearized analytical results. Furthermore, the standard errors for all variables were utilized to compute the goodness-of-fit for both error analyses [Hybrid fractional error (HYBRID)], the sum of squares of errors (ERRSQ), and the nonlinear chi-square test (X2). As a result, three various error functions were tested experimentally to choose the optimal isotherm model for the experimental data.
Hybrid fractional error
The hybrid fractional error (HYBRID) function is employed as it is more reliable than other error functions and adapts to low concentrations by optimizing absolute deviation versus fractional error. According to the literature, the hybrid error is given by Eq. (11) [50, 51].
where: qm represents the isotherm model constant, N represents the number of data points, P represents the number of isotherm parameters, qe represents the equilibrium capacity determined from experimental data, and q represents the average of qe.
The sum of the squares of the errors
The sum of the squares of the errors is given by Eq. (13) below (ERRSQ) [52].
Nonlinear chi-square test (χ2)
A nonlinear chi-square test is a statistical method for determining the optimum treatment strategy. The chi-square error (χ2) equation is given to be the next equation:
By comparing the error function values, it was discovered that the Freundlich isotherm theory is substantially greater than the other three equations. The Freundlich isotherm model has good correlation coefficients with low values for both Hybrid, ERRSQ, and chi-square, as seen in Table 3.
3.5 Kinetics modeling
To study the governing mechanisms of the sorption mechanism, such as mass transfer and chemical reaction, the pseudo-first-order and pseudo-second-order kinetics models, and the intraparticle diffusion model, were employed to assess the experimental adsorption by A. platensis [14].
3.5.1 Pseudo-first-order kinetics models
When the experimental results for dye elimination were determined using the pseudo-first-order (Eq. (5)), a perfectly straight line was obtained when a graph of ln (qe and qt) and t was produced, suggesting a non-fit on the equation (Fig. 12). For rigorous surface adsorption, the rate variation ought to be equal to the first power of concentration. The initial solute concentration and adsorption rate have a linear relationship because pore diffusion inhibits adsorption. The pseudo-first-order data suit the Lagergren model well, but the adsorption data deviate from the theory after that. As a consequence, the model covers the early phases of rapid adsorption but can be extended to include the complete adsorption process. Additionally, except at a concentration of 90 mg L−1, where the estimated qe values are equal to the observed qe values and the correlation coefficient R2 is rather high (Table 3), the adsorption of MO onto A. platensis is a first-order reaction. The number of pollutant-occupied adsorption sites on the adsorbent surface is compared to the number of unoccupied sites in the pseudo-first-order model [61].
3.5.2 Pseudo-second-order equation
According to the pseudo-second-order, the rate-limiting phase in chemical sorption is valance force through electron sharing or exchange between the adsorbent and adsorbate [62]. If second-order kinetics is applicable, a linear relationship between t/qt and t should be evident in the plot. The intercept and slope of the t/qt vs. t graphs were used to compute K2 and the equilibrium adsorption capacity qe (Fig. 13). With correlation coefficients higher than 0.991 for the second-order kinetic model, the linear plots of t/qt versus t at three initial MO concentrations (15, 90, and 150 mg L−1) demonstrate good agreement between experimental and calculated qe values. This demonstrates that the pseudo-second-order model provided a good correlation for the adsorption of varying initial MO dye concentrations. The pseudo-second-order rate constant (k2) reduced from 24.42 to 0.95 as the initial MO concentration increased from 15 to 90 mg L−1.
3.5.3 Intraparticle diffusion
Intra-particle diffusion is the rate-controlling factor in an adsorption system, adsorbate uptake differs with the square root of time [63]. Adsorption rates are usually evaluated by measuring the adsorbent’s adsorption capacity as a function of the square root of time. The relationship plot of qt and t1/2 obtained for MO adsorption on A. platensis nanoparticles in this study does not go to the origin, and two separate regions exist. As a consequence, Eq. (5) and Kdif and C values obtained from the slopes and intercepts of the plot’s linear parts were used to analyze two linear areas individually.
Table 4 and Fig. 14 show R2 values near unity, indicating that this model was used. The intercept C values (Table 4) indicate the thickness of the boundary layer; even as the intercept increases, so does its resistance to exterior mass transfer. The constant C was observed to increase from 0.15 to 48.41 with an increase in dye concentration from 15 to 150 mg L−1, indicating an increase in boundary layer thickness, a decrease in the likelihood of external mass transfer, and an increase in the likelihood of internal mass transfer [64, 65]. Higher initial MO dye concentrations were found to result in a decrease in the constant C, which suggests that the boundary layer thickness has decreased and the likelihood of external mass transfer has increased. Also, the intraparticle diffusion rate constant, Kdif, was in the 0.05–1.95 mg g−1 min−1/2 range and decreased as the initial dye concentration increased from 15 to 90 mg L−1. The plots’ linearity exposed that intraparticle diffusion played a substantial effect on adsorbate uptake through adsorbent [63].
3.6 Application of A. platensis in MO removal from wastewater
Wastewater having the parameters shown in Table 5, which was previously filtered with a Millipore 0.45 mm filter. To investigate the effect of A. platensis on MO removal, deionized water containing the same dye concentration as wastewater was generated as a control. A wastewater sample was collected from the El-Emoum drain, Alexandria, Egypt, and which comprises numerous industrial sewage and agricultural wastes.
The removal efficiency of MO by A. platensis was assessed using wastewater, and the results are shown in Table 5. In the existence of other metal ions, a dosage of 0.1 g of A. platensis was shown to be sufficient, with extraction efficiency of more than 24.6 and 11.5% for both seawater and wastewater, respectively. The removal efficiency of MO from wastewater increased to 99.24% when compared to the results obtained using deionized water.
This could be due to organic compounds in wastewater being adsorbed onto A. platensis and eliminating a portion of the binding. Organic material and ion complexation in the dye could also reduce MO dye adsorption on A. platensis. In any case, the outcomes demonstrate the viability of using A. platensis as a low-cost adsorbent to extract MO from wastewater. To further show the applicability of A. platensis in extracting MO dye from wastewater, Table 2 compares the maximum capacity of several adsorbents for MO published in the literature. Furthermore, A. platensis have a much better capacity than the other low-cost adsorbents, indicating that it could be a promising low-cost adsorbent used for industrial wastewater treatment.
Table 6 illustrates the adsorption performance A. platensis for MO removal capacity compared with other adsorbents and bio-adsorbents. The results revealed that effective adsorbent compared to various studies on MO removal.
3.7 Regeneration and reusability study
Industrial applications can benefit from regeneration studies on the possibility of desorbing MO molecules from A. platensis. The solvent desorption technique was used by adding 50 ml of the solution to MO-loaded A. platensis and shaking it at 150 rpm for 3 h at pH 1 and 5 mg L−1. The number of regeneration cycles elevated from one to three, and the effectiveness of MO dye adsorption from A. platensis in synthetic solution decreased from 97.73 to 89.28% (Fig. 15). This may be caused by the insufficient desorption of MO dye that A. platensis has absorbed. The regeneration result shows that A. platensis loses only 8.44% of its adsorbing capacity after three cycles for the synthetic solution due to the decreased biomass amount in the solution and available sites number for adsorption [66]. Furthermore, our research indicated that A. platensis can be regenerated and still had high adsorption effectiveness even after being utilized after three times. A saturated bio-adsorbent must be disposed of and managed in line with the needs of the relevant parties, and it must be marked as hazardous waste after it has significantly decreased in adsorption ability and is no longer reliable for use in the future [67].
3.8 Proposed adsorption mechanism
This study showed that the numerous functional groups on the surface of A. platensis biomass, including as –OH and –NH groups, C = O groups and aromatics groups, are substantially dependent on the adsorption of MO dye from aqueous solution by A. platensis biomass. Other research has found that brown algal cell walls contain alginate, a key polymer in blue-green algae algae that provides carboxyl groups. After protonation and deprotonation, the surface of A. platensis functional groups might be charged (negative and positive) or neutral. Besides, the electrostatic affinity between functional groups and the MO dye molecule may describe MO sorption on A. platensis biomass. Moreover, The average ∆G° value for the MO adsorption developed in this work is − 10.1 kJ mol−1, indicating that the dominating mechanism was physical adsorption via a physical process involving weak attraction forces.
4 Conclusions
The objective of this investigation was to see if A. platensis nanoparticles might be utilized as a natural and environmentally acceptable product to remove the methyl orange color from water. The study experimented with various factors such as contact time, initial dye concentration, adsorbent dose, and pH. It was found that the best results were achieved with a low pH of 1 and a concentration of 30 mg L−1. To better understand how the MO dye was being removed from the water by the A. platensis biomass, the adsorption mechanisms were investigated using several analytical techniques including FTIR, BET, and SEM. The results presented that the multipoint BET, single point BET surface area, and BJH adsorption cumulative micropore volume of the A. platensis was 139.83 m2 g─1, 114.373 m2 g−1, and 0.101 cc g─1, respectively. In a batch condition at 20 °C, the adsorption behavior was performed by kinetic and equilibrium isothermal studies. Moreover, the thermodynamic factors showed the exothermic nature and physisorption of the adsorption process, which tends to be spontaneous at lower temperatures. Freundlich, Langmuir, Temkin, and Dubinin-Radushkevich isotherm models were also performed to evaluate the removal of MO dye into nanoparticles of A. platensis. The Freundlich equation, which indicates multilayer adsorption on the heterogeneous surface, provided the best representation of the equilibrium adsorption data. The qe experiment and calculation values for the pseudo-first-order, pseudo-second-order, and interparticle diffusion kinetic models were determined. The results displayed that A. platensis exhibited 99% of MO dye removal. Furthermore, it will aid in the reusability of adsorbents, which may then be employed to adsorb MO dye ions and establish an effective adsorption process. Finally, our work revealed that A. platensis dried nanoparticles are an efficient and appealing biomaterial for MO dye adsorption from synthetic aqueous solution.
Data availability
Not applicable.
References
Blaber SJ, Cyrus D, Albaret J-J, Ching CV, Day J, Elliott M, Fonseca M, Hoss D, Orensanz J, Potter I (2000) Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. iCES J Marine Sci 57:590–602
Salman JM, Amrin AR, Hassan FM, Jouda SA (2015) Removal of congo red dye from aqueous solution by using natural materials. Mesopotamia Environ J 1:82–89
Reddy MS, Sivaramakrishna L, Reddy AV (2012) The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J Hazard Mater 203:118–127
Bazrafshan E, Zarei AA, Nadi H, Zazouli MA (2014) Adsorptive removal of methyl orange and reactive red 198 dyes by Moringa peregrina ash. Ind J Chem Technol 21:105–113
Seifpanahi Shabani K, Abedi-Orang B (2020) Experimental studies, response surface methodology and molecular modeling for optimization and mechanism analysis of methylene blue dye removal by different clays. J Min Environ 11:1079–1093
Mo JH, Lee YH, Kim J, Jeong JY, Jegal J (2008) Treatment of dye aqueous solutions using nanofiltration polyamide composite membranes for the dye wastewater reuse. Dyes Pigm 76:429–434
Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH (2016) Kinetic, isotherms and thermodynamic modeling for adsorption of Acid Blue 92 (AB92) from aqueous solution by modified Azolla filicoloides. Fresenius Environ Bull 25:1322–1331
Guo J, Qiu L, Deng Z, Yan F (2013) Plastic reusable pH indicator strips: preparation via anion-exchange of poly (ionic liquids) with anionic dyes. Polym Chem 4:1309–1312
ALzaydien AS (2009) Adsorption of methylene blue from aqueous solution onto a low-cost natural Jordanian Tripoli. Am J Appl Sci 6:1047
Abbas EM, Ali FS, Desouky MG, Ashour M, El-Shafei A, Maaty MM, Sharawy ZZ (2021) Novel comprehensive molecular and ecological study introducing coastal mud shrimp (Solenocera crassicornis) recorded at the Gulf of suez, Egypt. J Mar Sci Eng 9:1–13. https://doi.org/10.3390/jmse9010009
Sen TK, Afroze S, Ang H (2011) Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water Air Soil Pollut 218:499–515
Yagub MT, Sen TK, Ang H (2012) Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut 223:5267–5282
Al Prol AE (2019) Study of environmental concerns of dyes and recent textile effluents treatment technology: a review. Asian J Fish Aquat Res 3:1–18
Gupta V (2009) Application of low-cost adsorbents for dye removal–a review. J Environ Manage 90:2313–2342
Ozsoy F, Ozdilek B, Onder A, Ilgin P, Ozay H, Ozay O (2022) Graphene nanoplate incorporated Gelatin/poly (2-(Acryloyloxy) ethyl trimethylammonium chloride) composites hydrogel for highly effective removal of Alizarin Red S from aqueous solution. J Polym Res 29:481
Al-Saeedi SI, Ashour M, Alprol AE (n.d.) Adsorption of toxic dye using red seaweeds from synthetic aqueous solution and its application to industrial wastewater effluents. Front Marine Sci 10:1202362. https://doi.org/10.3389/fmars.2023.1202362
Rita K (2012) Adsorption of dye eosin from an aqueous solution on two different samples of activated carbon by static batch method. J Water Res Protect 4(2):17426. https://doi.org/10.4236/jwarp.2012.42011
Onder A, Ozay H (2021) Highly efficient removal of methyl orange from aqueous media by amine functional cyclotriphosphazene submicrospheres as reusable column packing material. Chem Eng Process-Process Intensif 165:108427
Rehman MSU, Kim I, Han J-I (2012) Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohyd Polym 90:1314–1322
Li T, Liu L, Zhang Z, Han Z (2020) Preparation of nanofibrous metal-organic framework filter for rapid adsorption and selective separation of cationic dye from aqueous solution. Sep Purif Technol 237:116360
Abdelrhman AM, Ashour M, Al-Zahaby MA, Sharawy ZZ, Nazmi H, Zaki MA, Ahmed NH, Ahmed SR, El-Haroun E, Van Doan H (2022) Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquacult Rep 25:101212. https://doi.org/10.1016/j.aqrep.2022.101212
Onder A, Kıvanç MR, Ilgin P, Ozay H, Ozay O (2023) Synthesis of p (HEMA-co-AETAC) nanocomposite hydrogel with vinyl-function montmorillonite nanoparticles and effective removal of methyl orange from aqueous solution. J Macromol Sci Part A 60:108–123
Shao W, Ebaid R, El-Sheekh M, Abomohra A, Eladel H (2019) Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): an overview. Grasas Aceites 70:e292–e292
Mansour AT, Alprol AE, Abualnaja KM, El-Beltagi HS, Ramadan KMA, Ashour M (2022) Dried brown seaweed’s phytoremediation potential for methylene blue dye removal from aquatic environments. Polymers 14(7):1375. https://doi.org/10.3390/polym14071375
Gaese H (2012) Chemical composition and potential application of Spirulina platensis biomass. Int J Agr Env 4:2012
Mabrouk MM, Ashour M, Labena A, Zaki MAA, Abdelhamid AF, Gewaily MS, Dawood MAO, Abualnaja KM, Ayoub HF (2022) Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia (Oreochromisniloticus) and its resistance to Aeromonashydrophila. Aquacult Res 53:125–135. https://doi.org/10.1111/are.15558
Mansour AT, Alprol AE, Abualnaja KM, El-Beltagi HS, Ramadan KMA, Ashour M (2022) The using of nanoparticles of microalgae in remediation of toxic dye from industrial wastewater: kinetic and isotherm studies. Materials (Basel) 15(11):3922. https://doi.org/10.3390/ma15113922
Sharawy ZZ, Ashour M, Labena A, Alsaqufi AS, Mansour AT (2022) Abbas EM Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp, Litopenaeus Vannamei. Aquaculture 551:737905. https://doi.org/10.1016/j.aquaculture.2022.737905
Mansour AT, Alprol AE, Ashour M, Ramadan KM, Alhajji AH, Abualnaja KM (2022) Do red seaweed nanoparticles enhance bioremediation capacity of toxic dyes from aqueous solution? Gels 8:310. https://doi.org/10.3390/gels8050310
Alprol AE, Heneash AMM, Ashour M, Abualnaja KM, Alhashmialameer D, Mansour AT, Sharawy ZZ, Abu-Saied MA, Abomohra AE (2021) Potential applications of Arthrospira platensis lipid-free biomass in bioremediation of organic dye from industrial textile effluents and its influence on marine rotifer (Brachionusplicatilis). Materials (Basel) 14(16):4446. https://doi.org/10.3390/ma14164446
Ashour M, Alprol AE, Heneash AMM, Saleh H, Abualnaja KM, Alhashmialameer D, Mansour AT (2021) Ammonia bioremediation from aquaculture wastewater effluents using Arthrospira platensis NIOF17/003: impact of biodiesel residue and potential of ammonia-loaded biomass as rotifer feed. Materials (Basel) 14:5460. https://doi.org/10.3390/ma14185460
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Alghamdi AA, Al-Odayni A-B, Saeed WS, Al-Kahtani A, Alharthi FA, Aouak T (2020) Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 2019:12
Temkin M, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim URSS 12:217–225
Fazal A, Rafique U (2012) Mechanistic understanding of cadmium sorption by sulfonated and esterified spent black tea. Int J 3:230–237
Ghoneim MM, El-Desoky HS, El-Moselhy KM, Amer A, Abou El-Naga EH, Mohamedein LI, Al-Prol AE (2014) Removal of cadmium from aqueous solution using marine green algae Ulva lactuca. Egypt J Aquat Res 40:235–242. https://doi.org/10.1016/j.ejar.2014.08.005Get
Dutta DP, Venugopalan R, Chopade S (2017) Manipulating carbon nanotubes for efficient removal of both cationic and anionic dyes from wastewater. ChemistrySelect 2:3878–3888
Padilha FP, de França FP, da Costa ACA (2005) The use of waste biomass of Sargassum sp. for the biosorption of copper from simulated semiconductor effluents. Bioresour Technol 96:1511–1517
Villaescusa I, Fiol N, Martı́nez MA, Miralles N, Poch J, Serarols J (2004) Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res 38:992–1002
Richner G, Puxty G (2012) Assessing the chemical speciation during CO2 absorption by aqueous amines using in situ FTIR. Ind Eng Chem Res 51:14317–14324
Celekli A, Yavuzatmaca M, Bozkurt H (2010) An eco-friendly process: predictive modelling of copper adsorption from aqueous solution on Spirulina platensis. J Hazard Mater 173:123–129
Adeyi AA, Jamil SNAM, Abdullah LC, Choong TSY, Lau KL, Abdullah M (2019) Adsorptive removal of methylene blue from aquatic environments using thiourea-modified poly (acrylonitrile-co-acrylic acid). Materials 12:1734
Pelosi B, Lima L, Vieira M (2014) Removal of the synthetic dye Remazol Brilliant Blue R from textile industry wastewaters by biosorption on the macrophyte Salvinia natans. Braz J Chem Eng 31:1035–1045
Gupta V, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Kareem A, Abd Alrazak N, Aljebori KH, Aljebori A, Algboory HL, Alkaim AF (2016) Removal of methylene blue dye from aqueous solutions by using activated carbon/ureaformaldehyde composite resin as an adsorbent. Int J Chem Sci 14:635–648
Bello OS, Ahmad MA (2011) Adsorptive removal of a synthetic textile dye using cocoa pod husks. Toxicol Environ Chem 93:1298–1308
Amuda OS, Olayiwola AO, Alade AO, Farombi AG, Adebisi SA (2014) Adsorption of methylene blue from aqueous solution using steam-activated carbon produced from Lantana camara stem. J Environ Prot 5:1352
Abualnaja KM, Alprol AE, Abu-Saied MA, Mansour AT, Ashour M (2021) Studying the adsorptive behavior of poly(acrylonitrile-co-styrene) and carbon nanotubes (nanocomposites) impregnated with adsorbent materials towards methyl orange dye. Nanomaterials (Basel) 11:1144. https://doi.org/10.3390/nano11051144
Kooh MRR, Dahri MK, Lim LB (2016) The removal of rhodamine B dye from aqueous solution using Casuarina equisetifolia needles as adsorbent. Cogent Environ Sci 2:1140553
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Coll Interface Sci 209:172–184
Iram M, Guo C, Guan Y, Ishfaq A, Liu H (2010) Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J Hazard Mater 181:1039–1050
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem 40:1347–1361
El-Bindary A, El-Sonbati A, Shoair A, Mohamed A (2015) Adsorptive removal of hazardous azorhodanine dye from an aqueous solution using rice straw fly ash. J Mater Environ Sci 6:1723–1732
Gupta V, Agarwal A, Singh M, Singh N (2017) Removal of red RB dye from aqueous solution by belpatra bark charcoal (BBC) adsorbent. J Mater Environ Sci 8:3654–3665
El-Bindary AA, Hussien MA, Diab MA, Eessa AM (2014) Adsorption of Acid Yellow 99 by polyacrylonitrile/activated carbon composite: kinetics, thermodynamics and isotherm studies. J Mol Liq 197:236–242
Al-Majedy YK, Al-Duhaidahawi DL, Al-Azawi KF, Al-Amiery AA, Kadhum AAH, Mohamad AB (2016) Coumarins as potential antioxidant agents complemented with suggested mechanisms and approved by molecular modeling studies. Molecules 21:135
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5:212–223
Abd El-Hamid HT, AlProl AE, Hafiz MA (2022) The efficiency of adsorption modelling and Plackett-Burman design for remediation of crystal violet by Sargassum latifolium. Biocatalysis Agric Biotechnol 44:102
Mansour AT, Alprol AE, Khedawy M, Abualnaja KM, Shalaby TA, Rayan G, Ramadan KM, Ashour M (2022) Green synthesis of zinc oxide nanoparticles using red seaweed for the elimination of organic toxic dye from an aqueous solution. Materials 15:5169. https://doi.org/10.3390/ma15155169
Abualnaja KM, Alprol AE, Abu-Saied MA, Ashour M, Mansour AT (2021) Removing of anionic dye from aqueous solutions by adsorption using of multiwalled carbon nanotubes and poly (acrylonitrile-styrene) impregnated with activated carbon. Sustainability 13:7077. https://doi.org/10.3390/su13137077
Sen TK, Sarzali MV (2008) Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminium oxide (Al2O3): a kinetic and equilibrium study. Chem Eng J 142:256–262
Wu Y, Zhang S, Guo X, Huang H (2008) Adsorption of chromium (III) on lignin. Biores Technol 99:7709–7715
El Nemr A (2009) Potential of pomegranate husk carbon for Cr (VI) removal from wastewater: kinetic and isotherm studies. J Hazard Mater 161:132–141
El Nemr A (2007) Pomegranate husk as an adsorbent in the removal of toxic chromium from wastewater. Chem Ecol 23:409–425
Boyd G, Schubert J, Adamson A (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. I. Ion-exchange equilibria1. J Am Chem Soc 69:2818–2829
Castañeda M, Mirasol M, Raymundo L, Solidum J (2012) Biosorption and desorption of lead (Pb+ 2) from simulated wastewater using freshwater snail shells, Melanoides tuberculata Muller (Family Thiaridae). In: 2nd International Conference on Environment and BioScience IPCBEE,(44). https://doi.org/10.7763/ipcbee
Lata S, Singh P, Samadder S (2015) Regeneration of adsorbents and recovery of heavy metals: a review. Int J Environ Sci Technol 12:1461–1478
Kamaru AA, Sani NS, Malek NANN (2016) Raw and surfactant-modified pineapple leaf as adsorbent for removal of methylene blue and methyl orange from aqueous solution. Desalin Water Treat 57:18836–18850
Lafi R, Hafiane A (2016) Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J Taiwan Inst Chem Eng 58:424–433
Subbaiah MV, Kim D-S (2016) Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: kinetics, isotherms, and thermodynamic studies. Ecotoxicol Environ Saf 128:109–117
Robati D, Mirza B, Rajabi M, Moradi O, Tyagi I, Agarwal S, Gupta V (2016) Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J 284:687–697
Banerjee D, Bhowmick P, Pahari D, Santra S, Sarkar S, Das B, Chattopadhyay K (2017) Pseudo first ordered adsorption of noxious textile dyes by low-temperature synthesized amorphous carbon nanotubes. Physica E 87:68–76
Zayed AM, Wahed MSA, Mohamed EA, Sillanpää M (2018) Insights on the role of organic matters of some Egyptian clays in methyl orange adsorption: isotherm and kinetic studies. Appl Clay Sci 166:49–60
Yu J, Zhang X, Wang D, Li P (2018) Adsorption of methyl orange dye onto biochar adsorbent prepared from chicken manure. Water Sci Technol 77:1303–1312
Zhang B, Wu Y, Cha L (2019) Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: performance, isotherm, and kinetic studies. J Dispers Sci Technol 41(1):125–136. https://doi.org/10.1080/01932691.2018.1561298
Lafi R, Abdellaoui L, Montasser I, Hafiane A (2022) Removal of methyl orange from aqueous solution onto modified extracted cellulose from Stipa Tenacissima L. Int J Environ Anal Chem 102:8124–8140
Abbou B, Lebkiri I, Ouaddari H, El Amri A, Achibat FE, Kadiri L, Ouass A, Lebkiri A (2023) Improved removal of methyl orange dye by adsorption using modified clay: combined experimental study using surface response methodology. Inorg Chem Commun 155:111127
Mon PP, Cho PP, Chandana L, Srikanth V, Madras G, Ch S (2023) Biowaste-derived Ni/NiO decorated-2D biochar for adsorption of methyl orange. J Environ Manage 344:118418
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to this work.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alprol, A.E., Khedawy, M., Ashour, M. et al. Arthrospira platensis nanoparticle-based approach for efficient removal of methyl orange dye from aqueous solutions: isotherm, kinetic, and thermodynamic analysis. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04844-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04844-z