Abstract
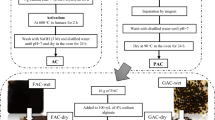
Contamination with pharmaceutical compounds, especially antibiotics, has become the focus of pollution control studies because of their high toxicity and difficulty in removing from aqueous media. In this study, activated carbon was prepared from Azolla filiculoides (ACAF) and magnetized using Fe3O4; finally, its further stabilization was done using ZnO nanoparticles (ACAF/Fe3O4/ZnO). The prepared adsorbent was used to remove ciprofloxacin (CIP) antibiotic from an aqueous solution. The adsorption–desorption process was performed in six consecutive runs, and only an 8% reduction was observed in the efficiency. Various parameters such as temperature, contact time, initial CIP concentration, nanocomposite concentration, and pH were examined. The results showed that removal of 100% was obtained at 75 min contact time for a CIP concentration of 10 mg/L at the optimum pH of 5 and temperature of 30 °C. The surface area and size of the nanocomposite were studied, which were equal to 1401 m2 g−1 and 2.26 nm, respectively. Also, the nanocomposite had a saturated magnetic property equal to 21.5 emu g−1. Equilibrium data were analyzed using four isothermal models and four kinetic models, and four error coefficient models were used to ensure. Due to the high regression coefficient and lower error coefficient, the Langmuir isotherm and pseudo-second-order (PSO) kinetic model were more consistent with equilibrium data. Moreover, the adsorption capacity of the adsorbent according to the Langmuir model was equal to 147.7, 153.3, 165.6, and 178.8 mg/g at temperatures of 20, 30, 40, and 50 °C. Examination of thermodynamic quantities shows that ΔG° is negative and ΔH° is positive. Therefore, the adsorption occurs optimally and spontaneously and has endothermic nature. Finally, this study shows a sustainable and commercially viable route for the use of environmentally friendly compounds.





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Balarak D, Mostafapour FK (2019) Photocatalytic degradation of amoxicillin using UV/Synthesized NiO from pharmaceutical wastewater. Indones J Chem 19(1):211–218
Vessally E, Musavi M, Poor Heravi M (2021) A Density functional theory study of adsorption ethionamide on the surface of the pristine, Si and Ga and Al-doped graphene. Iran J Chem Chem Eng 40(6):1720–1736. https://doi.org/10.30492/ijcce.2022.532176.4794
Carabineiro SAC, Thavorn-amornsri T, Pereira MFR, Figueiredo JL (2012) Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal Today 186:29–34
Carabineiro SAC, Thavorn-Amornsri T, Pereira MFR, Figueiredo JL (2011) Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res 45:4583–4591
Balarak D, Azarpira H, Mostafapour FK (2016) Adsorption kinetics and equilibrium of ciprofloxacin from aqeous solutions using Corylus avellana (Hazelnut) activated carbon. Int J Pharm Technol 8:16664–16675
Nassar MY, Ahmed IS, Raya MA (2019) A facile and tunable approach for synthesis of pure silica nanostructures from rice husk for the removal of ciprofloxacin drug from polluted aqueous solutions. J Mol Liq 282:251–263
Zhu X, Tsang DCW, Chen F, Li S, Yang X (2015) Ciprofloxacin adsorption on graphene and granular activated carbon: kinetics, isotherms, and effects of solution chemistry. Environ Technol 36:3094–3102
Yin D, Xu Z, Shi J, Shen L, He Z (2017) Adsorption characteristics of ciprofloxacin on the schorl: kinetics, thermodynamics, effect of metal ion and mechanisms. J Water Reuse Desal 8:350–359
Fakhri A, Adami S (2014) Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J Taiwan Inst Chem Eng 45:1001–1006
Balarak D, Mahvi AH, Shim MJ, Lee S-M (2020) Adsorption of ciprofloxacin from aqueous solution onto synthesized NiO: isotherm, kinetic and thermodynamic studies. Desalin Water Treat 203:1–11
Azarpira H, Mahdavi Y, Khaleghi O (2016) Thermodynamic studies on the removal of metronidazole antibiotic by multi-walled carbon nanotubes. Pharm Lett 8(11):107–113
Kumar A, Patra C, Kumar S, Narayanasamy S (2022) Effect of magnetization on the adsorptive removal of an emerging contaminant ciprofloxacin by magnetic acid activated carbon. Environ Res 206:112604
Giahi M, Rahbar A, Mehdizadeh K (2021) Photochemical degradation of an environmental pollutant by pure ZnO and MgO doped ZnO nanocatalysts. Iran J Chem Chem Eng 40(1):83–91. https://doi.org/10.30492/ijcce.2019.36825
Lakshman M (2022) Fe3O4@SiO2-Pip-SA nanocomposite: a novel and highly efficient reusable acidic catalyst for synthesis of rhodanine derivatives. J Synth Chem 1(1):48–51. https://doi.org/10.22034/jsc.2022.149234
Issa M (2022) Rapid enzymatically reduction of zincum gluconicum for the biomanufacturing of zinc oxide nanoparticles by mycoextracellular filtrate of penicillium digitatum (Pdig-B3) as a soft green technique. Arch Razi Inst 77(1):101–110. https://doi.org/10.22092/ari.2021.356422.1841
Mansouri M, Nademi M, Ebrahim Olya M, Lotfi H (2017) Study of methyl tert-butyl ether (MTBE) photocatalytic degradation with UV/TiO2-ZnO-CuO nanoparticles. J Chem Health Risks 7(1):19–32. https://doi.org/10.22034/jchr.2017.544161
Gupta S (2022) Magnetic nanoparticles supported sulfuric acid as a green and efficient nanocatalyst for oxidation of sulfides and oxidative coupling of thiols. J Synth Chem 1(1):16–21. https://doi.org/10.22034/jsc.2022.149217
Saravanan R, Gupta VK, Edgar M (2014) Gracia F (2014) Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J Mol Liq 198:409–412
Al-Khateeb LA, Almotiry S, Salam MA (2014) Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem Eng J 248:191–199
Jamshidi M, Ghaedi M, Dashtian K, Hajati S (2016) Bazrafshan AA (2016) Sonochemical assisted hydrothermal synthesis of ZnO: Cr nanoparticles loaded activated carbon for simultaneous ultrasound-assisted adsorption of ternary toxic organic dye: derivative spectrophotometric, optimization, kinetic and isotherm study. Ultrason Sonochem 32:119–131
Yilmaz M, Mengelizadeh N, Saloot MK, shahbaksh, S (2022) Facile synthesis of Fe3O4/ZnO/GO photocatalysts for decolorization of acid blue 113 under solar, visible and UV lights. Mater Sci Semicond Process 144:106593
Al-Musawi TJ, McKay G, Kadhim A (2022) Activated carbon prepared from hazelnut shell waste and magnetized by Fe3O4 nanoparticles for highly efficient adsorption of fluoride. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02593-z
Chandrasekar C, Rajendran HK, Priyan V, Narayanasamy S (2022) Valorization of sawdust by mineral acid assisted hydrothermal carbonization for the adsorptive removal of bisphenol A: a greener approach. Chemosphere 303:135171
Chitongo R, Opeolu BO, Olatunji OS (2019) Abatement of Amoxicillin, Ampicillin, and Chloramphenicol from aqueous solutions using activated carbon prepared from grape slurry. Clean Soil Air Water 47:1800077
Patra C, Gupta R, Bedadeep D, Narayanasamy S (2020) Surface treated acid-activated carbon for adsorption of anionic azo dyes from single and binary adsorptive systems: a detail insight. Environ Pollut 266:115102
Balarak D, Baniasadi M, Lee SM, Shim MJ (2021) Ciprofloxacin adsorption onto Azolla filiculoides activated carbon from aqueous solutions. Desalin Water Treat 218:444–453
Diyanati RA, Yousefi Z, Cherati JY (2013) The ability of Azolla and lemna minor biomass for adsorption of phenol from aqueous solutions. J Mazand Univ Med Sci 23:17–23
Zazouli MA, Mahvi AH, Dobaradaran S, Barafrashtehpour M (2014) Adsorption of fluoride from aqueous solution by modified Azolla Filiculoides. Fluoride 47(4):349–358
Diyanati RA, Yousefi Z, Cherati JY (2013) Investigating phenol adsorption from aqueous solution by dried azolla. J Mazand Univ Med Sci 22:13–21
Balarak D, Mahvi AH, Shahbaksh S, Wahab MA (2021) Abdala A (2021) Adsorptive removal of azithromycin antibiotic from aqueous solution by azolla filiculoides-based activated porous carbon. Nanomaterials 11(12):3281
Balarak D, Al-Musawi TJ, Mohammed IA et al (2020) The eradication of reactive black 5 dye liquid wastes using Azolla filiculoides aquatic fern as a good and an economical biosorption agent. SN Appl Sci 2:1015
Diao Y, Walawender WP, Fan LT (2002) Activated carbons prepared from phosphoric acid activation of grain sorghum. Bioresour Technol 81:45–52
Igwegbe CA, Oba SN, Aniagor CO, Adeniyi AG, Ighalo JO (2021) Adsorption of ciprofloxacin from water: a comprehensive review. J Ind Eng Chem 93:57–77
Rahdar A, Rahdar S, Ahmadi S, Fu J (2019) Adsorption of ciprofloxacin from aqueous environment by using synthesized nanoceria. Eco Chem Eng Sci 26(2):299–311
Dhiman N, Sharma N (2019) Batch adsorption studies on the removal of ciprofloxacin hydrochloride from aqueous solution using ZnO nanoparticles and groundnut (Arachis hypogaea) shell powder: a comparison. Indian Chem Eng 61(1):67–76
Sousa WRDN, Oliveira AR, Filho JFC, Dantas TCM, Santos AGD, Caldeira VPS, Geraldo EL (2018) Ciprofloxacin adsorption on ZnO supported on SBA-15. Water Air Soil Pollut 229:1–12
Tang Y, Guo H, Xiao L, Yu S, Gao N, Wang Y (2013) Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf A Physicochem Eng Asp 424:74–80
Chen H, Zhang Z, Feng M (2017) Degradation of 2,4-dichlorophenoxyacetic acid in water by persulfate activated with FeS (mackinawite). Chem Eng J 313:498–507
Khoshnamvand N, Ahmadi S, Mostafapour FK (2017) Kinetic and isotherm studies on ciprofloxacin an adsorption using magnesium oxide nanopartices. J Appl Pharm Sci 7(11):79–83
Kyzas GZ, McKay G, Al-Musawi, TJ, Salehi S, Balarak D (2022) Removal of benzene and toluene from synthetic wastewater by adsorption onto magnetic zeolitic imidazole framework nanocomposites. Nanomaterials 12:3049. https://doi.org/10.3390/nano12173049
Mahvi AH, Mostafapour FK (2018) Biosorption of tetracycline from aqueous solution by Azolla Filiculoides: equilibrium kinetic and thermodynamic studies. Fresenius Environ Bull 27:5759–5767
Balarak D, Azarpira H, Mostafapour FK (2016) Adsorption isotherm studies of tetracycline antibiotics from aqueous solutions by maize stalks as a cheap biosorbent. Int J Pharm Technol 8(3):16664–16675
Zhang H, Wang J, Zhou B, Zhou Y, Dai Z, Zhou Q (2018) Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: kinetics, isotherms and influencing factors. Environ Pollut 243:1550–1557
Ai Y, Liu Y, Huo Y (2019) Insights into the adsorption mechanism and dynamic behavior of tetracycline antibiotics on reduced graphene oxide (RGO) and graphene oxide (GO) materials. Environ Sci Nano 6:3336–3348
Yu F, Bai XT, Liang MX, Ma J (2021) Recent progress on metal-organic framework-derived porous carbon and its composite for pollutant adsorption from liquid phase. Chem Eng J 405:71–86
Ahmadi S, Banach A, Mostafapour FK (2017) Study survey of cupric oxide nanoparticles in removal efficiency of ciprofloxacin antibiotic from aqueous solution: adsorption isotherm study. Desalin Water Treat 89:297–303
Yu F, Li Y, Huang GQ, Yang CF, Chen C, Zhou T (2020) Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 260:127650
Popoola LT (2019) Tetracycline and sulfamethoxazole adsorption onto nanomagnetic walnut shell-rice husk: isotherm, kinetic, mechanistic and thermodynamic studies. Int J Environ Anal Chem 100:1–23
Balarak D, Azarpira H, Mostafapour FK (2016) Study of the adsorption mechanisms of cephalexin on to Azolla filiculoides. Pharm Chem 8:114–121
Adekola FA, Adegoke HI, Adebayo GB, Abdulsalam IO (2016) Batch sorption of ciprofloxacin on kaolinitic clay and nhematite composite: equilibrium and thermodynamics studies. Mor J Chem 4:384–424
Wang L, Chen G, Ling C, Zhang J, Szerlag K (2017) Adsorption of ciprofloxacin on to bamboo charcoal: effects of pH, salinity, cations, and phosphate. Environ Prog Sustain 36:1108–1115
Wu P, Cai Z, Jin H, Tang Y (2019) Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci Total Environ 650:671–678
Liu G, Zhu Z, Yang Y, Sun Y, Yu F, Ma J (2019) Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ Pollut 246:26–33
Yilmaz M, Al-Musawi TJ, Saloot MK (2022) Synthesis of activated carbon from Lemna minor plant and magnetized with iron (III) oxide magnetic nanoparticles and its application in removal of Ciprofloxacin. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-02279-y
Wang Z, Muhammad Y, Tang R, Lu C, Yu S, Song R (2021) Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep Purif Technol 274:119059
Ma J, Xiong Y, Dai X (2020) Adsorption behavior and mechanism of ciprofloxacin and Cu(II) on graphene hydrogel wetted surface. Chem Eng J 380:122387
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467
Jiang Z, Yu F, Ma J (2019) Design of graphene-based adsorbents and its removal of antibiotics in aqueous solution. Acta Phys Chim Sin 35:709–724
Shahnaz T, Bedadeep D, SelvarajuNarayanasamy S (2022) Investigation of the adsorptive removal of methylene blue using modified nanocellulose. Int J Biol Macromol 200:162–171
Patra C, Suganya E, Sivaprakasam S, Krishnamoorthy G, Narayanasamy S (2021) A detailed insight on fabricated porous chitosan in eliminating synthetic anionic dyes from single and multi-adsorptive systems with related studies. Chemosphere 2181:130706
Funding
This work was supported by Zahedan University of Medical Sciences, Zahedan, Iran (code: 10800). Dr. GS Zaman extend their appreciation to the Deanship of Scientific Research at King Khalid University, KSA, for funding this work through small research group program under grant number RGP.01/299/43.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Ameer A. Alameri, Raed H. C. Alfilh, Sameer A. Awad, and Gaffar Sarwar Zaman. The first draft of the manuscript was written by Davoud Balarak and Tariq J. Al–Musawi; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alameri, A.A., Alfilh, R.H.C., Awad, S.A. et al. Ciprofloxacin adsorption using magnetic and ZnO nanoparticles supported activated carbon derived from Azolla filiculoides biomass. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03372-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03372-6