Abstract
Great concern for human health has led the food industry to focus on functional products. Microparticles based on nanoemulsions (M1) and nanocomposites (M2) were developed to deliver vital agents against colorectal cancer and microbial infection. The functional microparticles were prepared by coating extra virgin olive oil (EVOO), probiotics, and fig leaves extract with sodium alginate (SA) and whey protein concentrate (WPC) using the freeze drying technique. The antimicrobial, cytotoxic, apoptotic, encapsulation efficiency (EE %), release rate, and antioxidant activity were investigated. The yogurt was loaded with microparticles and evaluated microbiology, chemically, and sensory during storage. The results showed that the size of nanoemulsion and nanocomposite was between 476.1 and 517.7 nm, while the zeta potentials were −30.1 and −34.5 mV, respectively. M2 microparticles recorded the lowest IC50 values against human colorectal cancerous Caco-2 and HCT 116 cell lines: 1.10 μg/mL and 15.34 μg/mL, respectively. The inhibition zones were between 11 to 20 and 9 to 18 mm for M1 and M2, respectively. The highest EE% was 89.20% for EVOO and 91.34% for probiotics in M2 microparticles. The induction period of the EVOO from M1 and M2 microparticles was 15.37 h and 13.09 h, respectively. The antioxidant activity was between 78 and 65.8% for M1 and M2 microparticles, respectively. The probiotics in yogurt with microparticles were more than un-coated cells, and the taste of these samples was acceptable during storage. This study suggests that microencapsulation could be considered an interesting therapeutic tool when EVOO and probiotics are used in functional food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Extra virgin olive oil (EVOO) has antiinflammatory, antioxidant, and anticancer activities [1]. These biological actions are associated with the high content of mono and polyunsaturated fatty acids and phenolic compounds of EVOO [2]. Despite these health benefits, one of the major problems of EVOO is storage oxidation, which leads to the formation of volatile compounds that cause undesirable flavor and free radicals [3].
Probiotics such as Lactobacillus and Bifidobacterium are considered vital microbes in the human intestine, and the supplementation of probiotic strains could positively impact the microbiome diversity and provide numerous health benefits to humans [4]. Baek and Lee [5] describe probiotics as live microbes that can afford a health benefit when administered in sufficient amounts by enhancing the intestinal microbial balance of the host. These health benefits include immunomodulation, anticancer effects, lowering cholesterol, improvement of lactose intolerance, reduction of antibiotic-associated diarrhea, and brain function modulation [6,7,8]. Many probiotic strains are sensitive to harsh conditions such as oxygen, gastric acids, and bile salts [9, 10]. Consequently, effective approaches are required to enhance the stability of probiotics in foods and during their passage through the gut.
Therefore, to obtain the maximum health value, reduce the EVOO storage oxidation process, and enhance the probiotic efficacy, two plans are applied. The first plan comprises antioxidant addition [11], and the second involves the microencapsulation process. The leaves of Ficus carica (known as Fig) have health benefits involving anticancer [12] and antimicrobial [13]. Fahimdanesh et al. [14] utilized the fig leaf extracts as a natural antioxidant to prevent olive oil oxidation. El-Sayed et al. [13] established that probiotics significantly propagated well when using fig leaf/leaves extract, especially with L. helveticus and L. rhamnosus. Microencapsulation is a method by which core materials (one or several) are surrounded by coating walls (single or double-layered) to form small capsules. The common polymer matrices used for EVOO and probiotic encapsulation are alginate, chitosan, gelatin, starch, and milk proteins [15, 16]. Many techniques have been developed to microencapsulate food ingredients, such as emulsification, extrusion, freeze-drying, and spray-drying. Bentonite is naturally occurring clay consisting of montmorillonite, a 2:1 type alumino silicate belonging to the smectite group [17]. Nanocomposite microcapsules are superior to polymeric nanocomposites in terms of surface structure, biodegradability, and chemical activity protection [18].
Hamed et al. [19] produced nanocomposite microspheres using clay/alginate/chitosan to function as delivery systems for omega-3-rich oils. Calvo et al. [1] studied the influence of different wall materials on the shelf-life of EVOO. Silva et al. [20] microencapsulated olive oil and lemon juice emulsion by freeze-drying to utilize an immediate sauce salad. Tee et al. [15] used freeze-drying, and extrusion techniques in encapsulating probiotics and obtained that freeze-drying is preferred due to a high bead production and probiotics survivability. Al-Furaih et al. [16] encapsulated probiotics with alginates using sodium chloride, olive oil, canola oil, and chitosan. Kowalska et al. [21] described various types of materials used for microencapsulation of probiotic bacteria to apply in the food industry. El-Sayed et al. [22] used stirred yogurt as a delivery model for synbiotic nanoemulsion and nanocomposite microcapsules. The objective of this study was to prepare microparticles based on functional nanoemulsion and nanocomposite using the freeze-drying technique. Also, the two prepared microparticles were assessed for encapsulation efficiency, oxidative stability, the release rate of EVOO, and probiotics. The anticancer and antimicrobial activities were also evaluated. In addition, functional yogurt was produced, where microbiology, chemical composition, and sensory analysis were studied as parameters of storage effect.
2 Materials and methods
2.1 Materials
Sodium alginate (SA) from brown algae (91%) was purchased from Loba chemia (India). Whey protein concentrate (WPC, 80 %) was received from Agri-mark, USA. Extra virgin olive oil (R1 with 0.15 % acidity value, 3.9 meq.O2/kg oil peroxide value, 191mg KOH/g saponification value, and 90.82 g I2/100g oil iodine value) was obtained from a local market, Giza, Egypt. Nano-clay hydrophilic bentonite and 2,2-diphenyl-1-picrylhydrazyl (DPPH, 90%), and 3-[4, 5-methylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO, USA). All other reagents and solvents were purchased from different suppliers and used without further purification. RNA Purification Kit and Revert Aid first-strand cDNA synthesis kit were purchased from (Thermo Fisher Scientific, USA). SYBR Green PCR kit was purchased from Qiagen (Valencia, CA, USA).
2.2 Microbial strains
Probiotic strains of Bifidobacterium lactis BB12 (provided by Food Science Dept. Penn State University USA) and Lacticaseibacillus rhamnosus NRRL B-442 (provided from Northern Regional Research Laboratory, NRRL) were individually activated using MRS broth [23]. This medium was inoculated with 2% lyophilized strains and anaerobically incubated at 37 °C for 48 h. The cells were obtained by centrifugation at 5000 rpm, for 15 min at 4 °C, and washed twice with sterile saline solution. The obtained cells of two probiotics (R2) were mixed and stored at 8 °C for further use.
The strains used in the antimicrobial activity test such as Salmonella typhimurium ATCC 14028, Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, Yersinia enterocolitica ATCC 23715, Listeria monocytogenes ATCC 5980, Aspergillus niger ATCC 10404, and Aspergillus flavus ATCC 9643 strains. All strains were collected from the Dairy Department, National Research Centre, Egypt.
2.3 Preparation of fig leaves/leaf extract
The dried fig leaves were milled with a special electric grinder (Moulinex) until obtaining powder form. One hundred grams of leaves/leaf powder was extracted at room temperature with 90% ethanol (500 mL, v/v) for 3 days. The extracted mixture was filtered and evaporated at 40 °C using a rotary evaporator as Fig. 1. The dried extract (R3) was collected and stored in dark, sterile screw-capped bottles at 4°C until future use.
2.4 Preparation of freeze-dried nanoemulsion and nanocomposite microparticles
Microencapsulation of nanoemulsion and nanocomposite was carried out based on emulsification, ultra-sonication, and freeze-drying techniques. Firstly, fig-nanoemulsion biopolymers were prepared by using an aqueous phase (distilled water), an oil phase (EVOO, 10% containing 2% of Tween 80), and fig leaves extract (4 mg/mL). The fig-coarse emulsion was obtained by homogenization with a high-speed homogenizer (X520, CAT Ingenieurburo M. Zipperer, GmbH, Germany) at 16,000 rpm for 3 min. Then, the prepared fig-emulsion was ultrasonically processed using a bench-scale sonicator (Newtown, CT, USA) with full power amplitude (60 W) for 10 min and 22.5 mm of probe diameter. The probiotic suspension was added to the fig-nanoemulsion with gentle stirring. Three percent of SA solution was prepared by dissolving it into deionized powder. An aqueous solution of WPC was prepared by dispersing 3 g of WPC powder into deionized water. SA and WPC solutions (in a ratio of 1:1) were mixed and stored overnight at room temperature for complete hydration of biopolymers. The resultant fig-nanoemulsion was added to the previously prepared biopolymers (SA-WPC).
The same procedure was used for the preparation of fig-nanocomposite biopolymers. Two grams of nanoclay hydrophilic bentonite was suspended in 100 mL of distilled water and stirred overnight (30 °C). Fifty milliliters of nanoclay suspension was mixed with the pre-prepared fig-emulsion for 2 h using a mechanical stirrer, followed by the ultra-sonication process. The fig-nanoemulsion and fig-nanocomposite biopolymers were frozen at −80 °C for 24 h. The freeze-drying technique (ALPHA 1-4 LSC) has been used in optimal conditions to obtain maximum probiotic survivability. After 48 h, freeze-dried fig-nanoemulsion and fig-nanocomposite biopolymers (M1 and M2) were ground by a planetary ball mill. The M1 and M2 microparticles were packed in sterile bottles (250 mL) and stored at 4 °C.
2.5 Antimicrobial activity
The antimicrobial activity of raw materials (R1, R2, and R3) and different microparticles (M1 and M2) was carried out according to El-Sayed and El-Sayed [24] using the well diffusion agar method. The tested strains were first activated by striking on Mueller–Hinton agar medium. After incubation overnight at 37 °C for bacteria and 72 h at 30 °C for fungi, they were picked from the agar medium surface. Typical colonies were introduced in 5 mL of tryptone soya broth and incubated at the same temperature until turbidity reached 0.5 “McFarland” standard solution. After that, 20 μL from each strain was swabbed on the surface of the hardened Mueller–Hinton agar medium on different plates. After resting for 20 min, the plates were well prepared in agar medium with a cropper (about 5 mm). Each well was filled with 100 μL of raw materials (R1, R2, and R3) and the prepared microparticles (M1 and M2). But the only EVOO (R1) was dissolved in dimethyl sulfoxide solution (DMSO) to facilitate the diffusion into agar medium (about 1 to1 concentration). The diameter of the inhibition zone was determined after incubation for 24 h at 37 °C for bacteria and after 72 h at 30 °C for fungi.
2.6 Anticancer activity
2.6.1 Cell culture, maintenance, and sub-culture
Human cell lines, including human colorectal HCT 116 and Caco-2 malignant cell lines, were obtained from VCSERA, which purchased them from the American Type Culture Collection (ATCC, USA). Dulbecco’s modified Eagle’s medium (DMEM) was used to cultivate them. Glucose with L-glutamine (4.5 g/L) (Lonza Bioproducts, Belgium) and 10% fetal bovine serum (FBS) were added to the medium (Seralab, UK). For growth maintenance, the cells were incubated in 5% CO2 humidified at 37 °C.
2.6.2 Cell proliferation by MTT assay
The viable cell percentages of human colorectal HCT 116 and Caco-2 cancerous cell lines after treatment with different concentrations of R1, R2, and R3 and the prepared microparticles (M1 and M2) were evaluated. These formulations were evaluated by the MTT assay as reported previously [25], with slight modification. In each well of the 96-well plate, 10,000 human colorectal cancer cells were seeded and allowed to attach overnight. On the following day, the whole medium was replaced with a new one, and each cell line was tested with various concentrations (0, 25, 50, and 100 μg/mL) of the produced formulations. A typical anticancer medicine, 5-fluorouracil (5-FU), was employed as a positive control and was given in the same dosages (0, 25, 50, and 100 μg/mL). The cells were then allowed to propagate for 24 h. Ten milliliters of 5 mg/mL MTT was applied to each well 4 h before the incubation period ended. After the incubation period was completed, 100 mL of DMSO was added to each well and left for 20 min. After the reaction, color development was measured at 450 nm using a Bio-Tek microplate reader.
2.6.3 Half-maximal inhibitory concentration measurement
The half-maximal inhibitory (IC50) values, which are the concentrations that inhibit 50% of HCT 116 and Caco-2 cancer cell viabilities, and fold changes of the microparticles (M1 and M2) versus 5-FU or their raw materials (R1, R2, and R3) were got by plotting the percentages of cell viabilities versus the concentrations of the test using polynomial concentration-response curve fitting models (Origin Pro 8 software).
2.6.4 Real-time qRT-PCR of p53, Bax, and Bcl-2 genes
Total RNA extraction
Total RNA was extracted from HCT 116 or Caco-2 cancerous cell lines. A million cells of each line were seeded and treated for 24 h with the IC50 dosage of M2 microparticles, the most efficient anticancer formula based on the results of the cytotoxic experiments, versus 5-FU, the standard anticancer drug against colorectal cancer. These cells were sub-cultured, and RNA was extracted using an RNA purification kit, as directed by the manufacturer.
Conversion of RNA to cDNA
First-strand cDNA was produced with 1 g of total RNA according to the manufacturer’s instructions using a Revert Aid First-strand cDNA synthesis kit. The antiapoptotic gene Bcl-2, the pro-apoptotic gene Bax, and the regulator/master gene of apoptosis (p53) were all measured in these samples. The SYBR Green PCR, as well as the forward and reverse primers for each gene, was used in quantitative real-time PCR using the DT-lite Real-Time PCR System (English/Russian system). In a total volume of 20 μL, the real-time PCR mixture contained 10 μL of SYBR PCR Master Mix, 1 μL of each primer (400 nM), 1 μL of cDNA, and 7 μL of RNase-free water. The initial activation temperature was 95 °C for 15 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 30 s. After real-time PCR, melting curves were used to illustrate the precise amplification of single products of interest. The amplification efficiency of the primers employed was determined using a standard curve test. A standard curve assay was done, and fold changes were accomplished using the comparative 2−ΔΔCt with the GAPDH house keeping gene (Table 1).
2.7 Particle size and the zeta potential of nanoemulsion and nanocomposite
Measurement of nanoemulsion and nanocomposite size and zeta potential was performed by a dynamic light scattering (DLS) method using Zeta sizer Nano ZS (Malvern Instruments, UK) at room temperature. Before measurements, 30 μL of the nanoemulsion and nanocomposite was diluted with 3 mL of water at 25 °C. Particle-size data were expressed as the mean of three independent batches of the nanoemulsion and nanocomposite.
2.8 Morphological investigations
Transmission electron microscope (TEM) analysis was carried out to study the morphology of the fig-nanoemulsion or nanocomposite biopolymers by using a JEOL JEM-1230 TEM. Twenty microliters of diluted samples was placed on a film-coated 200-mesh copper specimen grid for 10 min, and the fluid excess was eliminated using filter paper. The grid was then stained with one drop of 3% phosphotungstic acid (PTA) and allowed to dry for 3 min. The coated grid was dried and examined under the TEM microscope (Philips, CM12). Then, samples were observed by operating at 120 kV. The morphology of the developed microparticles (M1 and M2) was also determined by a scanning electron microscope (SEM) (quanta Fei 250, republic Czech).
2.9 Rancimat measurements
A Methrom Rancimat model 892 (Herisau, Switzerland) was used to measure the oxidative stability of EVOO as the reference investigation for all olive oil samples. The extracted EVOO from M1 and M2 microparticles was determined as the induction period (IP), which was expressed in hours (h). The oxidation reactions were increased by keeping 3 g of oil at 110 °C under a steady airflow of 20 L/h. The conductivity variation of 60 mL of water was detected due to an increase in volatile oxidative compounds.
2.10 Encapsulation efficiency
The EVOO encapsulation efficiency (EE) was calculated from Eq. 1 according to a method described by Carneiro et al. [26]. The total oil content is supposed to be equivalent to the initial oil since preliminary investigations have revealed that all the initial oil was retained, which was expected since the EVOO is non-volatile. The surface oil (non-encapsulated) was detected from the mass difference between the initial clean flask and that having the extracted oil [27]. Briefly, 50 mL of n-hexane was added to 2 g of microparticle powder in a glass jar with a lid, followed by shaking by hand for 2 min for the extraction of free oil. The solvent mixture was then filtered through Whatman no. 1 filter paper, and the powder residue was rinsed three times with n-hexane (20 mL). Then, the solvent was evaporated at 60 °C until constant weight at reduced pressure was achieved [26].
The encapsulation efficiency of probiotics in resulting microparticles was calculated according to Mahmoud et al. [28]. One gram of the different microparticles was dissolved in a tri-sodium citrate solution (2%, w/v) and followed with serially diluted by saline solution. The suitable dilution was poured using MRS agar plates and anaerobically incubated at 37 °C for 48 h. The encapsulation probiotic efficiency (EE %) was calculated as the following equation (Eq. 2) [28]:
where N is the number of the released probiotic cells from the microparticles, and No is the number of the added probiotic cells to different formulations.
2.11 Survivability for probiotic cells of microparticles
The viability of probiotics in un-coated cells and M1 and M2 microparticles in an acidic gastric solution was determined according to Mahmoud et al. [28]. The acidic human stomach conditions solution was prepared by adding 0.3% pepsin enzyme to a 0.9% sodium chloride (NaCl) solution and adjusting the pH to 2.0. Un-coated cells and M1 and M2 microparticles were added to dialysis bags individually and incubated in a prepared stomach solution for 2 h. After that time, the un-coated cells and M1 and M2 microparticles were transferred to the intestine solution. This solution was prepared by adding 1% pancreatic enzyme to 0.65% sodium chloride (NaCl), 0.0835% potassium chloride (KCl), 0.022% calcium chloride (CaCl2), 0.1386% sodium bicarbonate (NaHCO3), and 0.3% bile salt and adjusting the pH to 7.5. The un-coated cells and M1 and M2 microparticles were incubated in an intestinal solution for 6 h. The viable counts were determined every 2 h intervals by dissolving one gram of suspended probiotics in 3.0% sodium citrate and pouring it into MRS agar plates. After incubation for 48h at 37 °C anaerobically, the colonies were counted and expressed as log CFU/g.
2.12 Release studies
For the EVOO release study, 1 g of microparticles was put into a dialysis bag and added to 100 mL of gastric and intestinal solutions at 37 °C under gentle stirring at 200 rpm. At specific time points (0, 1, 2, 3, 4, 5, and 6 h), 3 mL of release medium was withdrawn and replenished with 3 mL of fresh medium. The amount of EVOO released was analyzed using a UV–Vis spectrophotometer at 235 nm as the highest absorption wavelength for the EVOO.
The release of probiotics from the microparticles was determined by Lotfipour et al. [29]. Ten grams of each un-coated cell and M1 and M2 microparticles was put in a dialysis bag individually and transferred into 100 mL of stimulating colonic solution (0.1 M potassium dihydrogen phosphate (KH2PO4), pH 7.0). One milliliter of each 1-h interval was taken, and the viable counts were enumerated using pour MRS agar plates and incubated at 37 °C for 48 h, anaerobically.
2.13 Production of functional yogurt
Fresh cow’s milk was pasteurized at 80 °C for 15 min and cooled to 42 °C [30]. Two percent of the yogurt starter cultures (L. bulgaricus and S. thermophilus) were added to pasteurized milk. After that, the inoculated milk was divided into three treatments. Treatment 1 (T1cells) was fortified with 2% of non-coated probiotic cells. Treatment 2 (T2M1) was fortified with 2% of M1 microparticles, and treatment 3 (T3M2) was fortified with 2% of M2 microparticles. All treatments were packed in plastic cups (100 mL) and incubated at 42 °C for 3 h until coagulated. After the incubation time, all treatment cups were stored in cool conditions for analysis for 21 days. The resulting yogurt treatments were analyzed microbiologically for probiotic counts, starter culture counts, aerobic bacterial counts, and fungi and mold counts using different growth media according to IDF [31] and APHA [32].
2.14 Antioxidant activity
The free radical scavenging assay of R1, R2, R3, M1, M2, T2M1, and T3M2 was determined according to the method described by Hamed et al. [19]. One gram of each sample was dispersed in 100 mL methanol for 1 h and then filtered using Whatman no. 1 filter paper. The powder residue was re-extracted twice with methanol (100 mL) for 15 min. Then, 100 μL of the sample (except fig extract 10 μL) filtrate was added to 900 μL of DPPH solution (0.0013% in methanol). The mixture was vigorously shaken and incubated for 30 min in the dark at room temperature. After incubation, the absorbance of each sample (Asample) was recorded at 517 nm against a blank (methanol) using a UV–Vis spectrophotometer (T80 UV/VIS Spectrometer, PG Instruments Ltd, UK). The absorbance control (Acontrol) was prepared with 100 μL of methanol and 900 μL of DPPH (i.e., at 100%). The free radical scavenging assay of each sample was expressed as the disappearance of the initial purple color. All measurements were performed in triplicate and reported as the average value. The percentage of free radical-scavenging capacity was calculated by the following formula (Eq. 3) [19]:
2.15 Chemical analysis and sensory evaluation for yogurt
The chemical analysis for pH, protein, fat, ash, and acidity was evaluated according to Ling [33] and AOAC [34]. The sensory evaluation was conducted according to Davis [35]. For sensory analysis, 20 members of Dairy Department, National Research Centre, Giza, Egypt, evaluated the yogurt during storage for flavor (50 points), appearance (10 points), and body and texture (40 points).
2.16 Statistical analysis
The results for cheese samples were analyzed according to the Statistical Analysis System Users Guide SAS (SAS Institute, Inc., USA) [36]. All experiments in this study were prepared in triplicate and mean values. Differences were measured significantly at p <0.05.
3 Results and discussion
3.1 Nanoemulsion and nanocomposite size and stability
The nanoemulsion and nanocomposite were prepared using the ultra-sonication process. Both particle size and stability were examined using a DLS technique. The mean diameter of the nanoemulsion and nanocomposite was 476.1 and 517.7 nm, respectively. It was noted that nanocomposite demonstrated a larger size than nanoemulsion. This may be attributed to the presence of clay. The same observation was shown by Peng et al. [37], in which the size of the nanoemulsion after the addition of montmorillonites became slightly bigger than the nanoemulsion without addition. Nciri et al. [38] also studied the clay addition effect on the olive oil properties in water emulsions. The poly dispersity index (PDI) was found to be 0.389 and 0.465 for the nanoemulsion and nanocomposite, respectively. The electrokinetic potential of prepared formulations was measured using the zeta potential [39]. Results indicated that the zeta potential of nanoemulsion and nanocomposite was found to be between −30.1 and −34.5 mV, confirming the stability of the formulations. It was noted that the zeta potential of the nanocomposite showed a slight increase due to the clay addition. These results are in the same line as Peng et al. [37]. The formulation system is considered stable if the zeta potential value is greater than +25 mV and less than 25 mV [39]. This is a result of the ability of particle surface charge to increase the stability of formulation through electrostatic repulsion [40].
3.2 Antimicrobial activity
Table 2 shows the susceptibility of pathogenic strains to raw materials (R1–R3) and different microparticles (M1 and M2) by well diffusion assay. The data demonstrated that R2 and R3 had inhibitory activities against tested strains by detecting inhibition zones. R2 has an inhibition zone that ranges between 7 and 18 mm against all tested strains. The same behavior was observed for R3, which gave an inhibition zone ranging between 5 and 10 mm, but the R1 did not have inhibitory actions. In the case of microparticles, the data indicated that the zones of inhibition were improved, especially for M1 more than M2 microparticles. The M1 microparticles gave an inhibition zone ranging between 11 and 20 mm, but the M2 microparticles showed an inhibition zone ranging between 9 and 18 mm. In the M2 microparticles, the probiotic cells or fig leaves extracts may be aggregated and adhered to the surface of the nanoclay which slows down the inhibitory action against tested strains as agreed by Li et al. [41]. Additionally, the more sustainable strain was Staphylococcus aureus, and other stains have a degree of inhibition in the same manner and range. Previous research has established that the antimicrobial activity of plant extract is generally related to the presence of alkaloids, flavonoids, and phenolic compounds isolated from fig leaves [13, 42, 43]. Also, others studied confirmed the antimicrobial activity of different Lactobacilli and Bifidobacterium strains that was related to their production of inhibitory peptides such as bacteriocins and hydrogen peroxide or different organic acids [44,45,46]. Consequently, the effect of inhibition activities for different microcapsules was related to the presence of fig leaves extract and the probiotic strains as mentioned by other authors Rostam et al. [47] and Mahmoudi et al. [48] who indicated that the extract of fig leaves had antimicrobial activities, and this inhibition action was independent of the pathogenic strains and the presence of phenolic compounds in the extract. Also, Denkova et al. [49] found that probiotic strains had antimicrobial against pathogens, in which this effect was related to the production and organic acids or the secretion of antimicrobial substances.
3.3 Anticancer activity
In the current study, the R1, R2, R3, and their newly twosynthesizedmicroparticles (M1 and M2) were examined in terms of their anticancer activities in vitro through testing their cytotoxic activities against human colorectal HCT116 and Caco-2 cancerous cell lines using MTT assay. The current results demonstrated that there was a gradual significant decrease (P > 0.05) in cell proliferation after treating the two proposed colorectalcancer cell lines (Caco-2 and HCT 116 cells) with R1, R2, R3, M1, and M2 using different dosages (0, 25, 50, and 100 μg/mL), as shown in Fig. 2A. The zero concentration represents the untreated colorectal cancerous cells and was used as a negative control. 5-Fluorouracil (5-FU), a standard anticancer drug, was used as a positive control and administratedinsimilardosages of the raw materials and microparticles. Figure 2B shows that the prepared formulationsaffected Caco-2 cells more than HCT 116 cells in terms of cytotoxicity, especially upon M2 application. Thus, we can suggest that HCT 116 cancerous cells were resistant to the applied formulations and 5-FU, the positive control, while Caco-2 cancerous cells were sensitive to them. Moreover, M1 microparticles also recorded high anticancer effects and low IC50 values against human colorectal cancerous Caco-2 and HCT 116 cell lines, 59.51 μg/mL and 78.65 μg/mL, respectively. The anticancer effect of M1 is relatively similar tothat of the standard drug, 5-FU. The fold change of M1 microparticleson Caco-2 cells versus 5-FU shows 0.98 among all treatments, while the fold change of M1 microparticles on HCT 116 cells versus 5-FU shows 0.83, as shown in Table 3.
Also, M2 microparticles recorded the highest cytotoxic effect and the lowest IC50 values upon Caco-2 and HCT 116 cells, 1.10 μg/mL and 15.34 μg/mL, respectively. The fold change of M2 microparticles on Caco-2 cells versus 5-FU shows the highest value (53.41) among all treatments, while the fold change of M2 microparticles on HCT 116 cells versus 5-FU shows a high value (4.27), as shown in Table 3.
The reason that M2 microparticles were significantly higher than M1 microparticles in their anticolorectal cancer effect may be due to the presence of clay in M2, not M1 microparticles. Firstly, it was suggested that the introduction of clay in the particles provides an adhesion force between the treated particles and the cancerous cells. This force represented by van der Waals and electrostatic attractions increases the cohesive energy density of that cancerous area when treated with clay-based particles. The increase in this energy after clay sorption on cell-cell and cell-extracellular matrix led to approaching the strategy of preventing cancer invasiveness [50]. Secondly, this adhesion strategy also allows the M2 microparticles to release the triple therapy of EVOO, fig, and probiotics locally and directly into the site of interest, either between cell and cell or cell–extracellular matrix. This promising strategy could provide a synergistic effect between the loaded natural agents in the M2 microparticles. The synergy between these natural agents produces an effect that is greater than the effect that would have resulted from adding each agent, which boosts the eradication of colorectal cancerous cells. Thirdly, it was reported that to avoid the interfering effect of two or more functional anticancer treatments loaded in a nanocarrier, clay-based nanocomposites might be used to provide an efficient synergistic effect with bi- or tri-functional delivery [51].
It was also reported that clay-based nanocompositeshave provided the best anticancer activity, using MTT assay “parallel to our results” and cell cycle arrest at S-phase, compared to other formulations. The anticancer activity results revealed that clay-based nanocomposite recorded significantly higher cytotoxicity than free counterparts on human breast cancerous MCF-7 cells [52]. These results are in agreement with ours, where M2 microparticles were significantly higher in cytotoxicity than R1, R2, and R3 on both colorectal cancerous cell lines (Fig. 2; Table 3). When comparing the fold change of M2 with M1 and M2 microparticles recorded the highest fold change versus M1 microparticles upon Caco-2 and HCT 116 cells, 54.33 and 5.12, respectively (Table 3).
3.4 Genetic expression
After studying the cytotoxic effect of the proposed formulations against human colorectal cancerous cells, this section investigates some mitochondrial apoptotic genes to prove the mechanistic way by which M2 microparticles reported the highest cytotoxic impact on both tested human colorectal cancerous cells. Noting that MTT dye, which was used in the cytotoxicity experiments, is a mitochondrial dye, we herein in this genetic section tracked the mitochondrial apoptotic Bcl-2, Bax, and p53 genes. These three genes are antiapoptotic, pro-apoptotic, and regulator/master gene of mitochondrial apoptosis, respectively. The mRNA genetic expressions of these genes were detected using real-time qPCR. Data in Fig. 3 indicated that M2 and 5-FU versus untreated cells “control” showed upregulation of p53 and Bax genetic expression levels and downregulation of Bcl-2 genetic expression compared to the untreated control cells. The M2 microparticles showed the highest effects with a very high significance value (p > 0.01), especially in Caco-2 cells, which were the most responsive to the treatment compared to HCT 116 cells, as previously shown in the cytotoxic evaluations.
The ratio of genetic expressions of pro-apoptotic (Bax) and antiapoptotic (Bcl-2) is very important to confirm the apoptotic effect of any new treatment. Therefore, this ratio was calculated in the current study and is shown in Table 4. The data indicates that the M2 microparticles have the highest Bax/Bcl-2 ratios, especially for Caco-2 cells (169.00) followed by HCT 116 cells (73.00) compared to control (1). Furthermore, 5-FU recorded high Bax/Bcl-2 ratios for HCT 116 and Caco-2 cell lines, 6.77 and 9.32, respectively, compared to control (1).
Intriguingly, our newly synthesized M2 microparticles recorded significantly higher Bax/Bcl-2 ratios than the standard drug 5-FU on both human colorectal cancer cell lines. Similar to results by Abd-Rabou and Edris [53] reported in that microparticles (M2) reported significantly higher inhibition of human hepatocellular carcinoma cell proliferation than free counterparts and the reference drug Doxorubicin. In addition, genetic expressions of pro-apoptotic (Bax) and antiapoptotic (Bcl-2) gene markers evaluated revealed that this M2 has better activity in Bax upregulation and Bcl-2 downregulation with the highest Bax/Bcl-2 ratio against cancerous cells [53]. Moreover, our results suggested that the anticancer power of the proposed microparticles, especially M2, may be caused by the controlled release of the phenolic agents and polyunsaturated fatty acids that are present in the encapsulated EVOO [2]. Therefore, EVOO got attention last year as a promising anticancer candidate [1]. In addition, EVOO formulations show anticancer activity due to the presence of high contents of antioxidants [54]. Pampaloni et al. [55] showed that the EVOO extracts have an antiproliferative effect on the cells of colon cancer through their interaction with estrogen-dependent signals involved in tumor cell growth. In addition to EVOO, probiotics can afford health benefits including immunomodulation and anticancer effect [8]. Finally, providing Fig to EVOO and probiotics enhanced the anticancer power of the newly synthesized M2 microparticles.
3.5 Encapsulation efficiency
Encapsulation efficiency (EE) is the most essential factor for determining the success of oil encapsulation, which is an indicator of coated and surface oil content. The encapsulation efficiency mainly depends on the used wall composition and its structure. It is also a measure of the ability of the wall material to protect the olive oil droplets. The EE of M1 microparticles was 74.66%. While in the case of M2 microparticles, the EE was 84.83%. The high value of EE (%) for M2 may be related to the perfect dispersion of this olive oil in the clay layers, consequently increasing its encapsulation. Singha and Hedenqvist [56] stated that the platelet structure of nanoclay enhanced the barrier properties of the coating composition. Koç et al. [57] studied the effect of the composition of wall materials on the microencapsulation of extra virgin olive oil using the spray drying method.
EE was also considered an important factor for evaluating the achievement of coated probiotics, and it determined the entrapped probiotic cell counts. Overall, the prepared microparticles showed the best efficiency results. In the case of the M2 microcapsule, the EE was recorded at 91.34%, while in the M1 microcapsule, it was recorded at 89.20%. It can be indicated that the EE percent was affected by the addition of nanoclay. So, the presence of nanoclay with biopolymer SA-WPC solutions could enhance the protection of probiotics inside microparticles by decreasing the pore size found in the capsulation network. Zhang et al. [58] found that the pore size and pore density could be controlled by changing the ratio of core material to shell material. Also, Jyothi et al. [59] indicated that high concentrations of polymers were given high microencapsulation efficiency. Generally, high viscosity and fast solidification of the materials agents contributed to reducing the porosity of microparticles [60].
3.6 Oxidative stability
The oxidation process of the extracted oil from microparticles was evaluated by monitoring the formation time of secondary oxidation products using the Rancimat method. The highest increase in oxidative stability was estimated by the induction period (IP). The IP of extracted oil from M1 microparticles (15.37 h) is higher than the IP of M2 microparticles (13.09 h). This could be due to the addition of clay that may adsorb the phenolic compounds found in the olive oil during the extraction process. As mentioned by Allaoui et al. [61], the polyphenols were recovered after adsorption onto natural clay using various solvents. Nciri et al. [38] studied the interaction of clay particles with olive oil and their adsorption at the interface. The same study summarized that clay particles had a major impact on the physicochemical characteristics of an olive oil–water emulsion. These findings confirmed our results that the addition of clay affects the olive oil microparticles. Phenolic compounds have high antioxidant activity that supports the resistance of olive oil against the oxidation process [62].
3.7 Morphological characterization
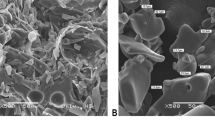
The morphology of the fig-nanoemulsion and nanocomposite biopolymers before the microencapsulation was revealed by TEM. The rod shape of probiotic bacteria appeared in the nanoemulsion and nanocomposite matrix (Fig. 4A and B). For the M2 microparticles, it can be noted that probiotic bacteria and oil droplets are well dispersed in the sheets of platelet clay layers. Also, the oil droplets aggregated into larger spherical clusters (Fig. 4B). As observed by Hamed et al. [19], there is a relative assemble of oil droplets in prepared nanocomposites without curcumin addition. The green color of fig leaf extracts changes the color of nanoemulsion and nanocomposite microparticles (M1 and M2) to greenish. With visual observation, the greenish microparticles were produced using the freeze-drying technique. The morphology of the developed microparticle process was shown by SEM. The M1 microparticles showed the rod shape of probiotic bacteria, as apparent from TEM photographs (Fig. 4C). Hao et al. [63] studied the morphology of three lactic acid bacteria and showed that the tested strains had cellular shapes using SEM examination. The microparticles have wrinkled, irregular forms with a wide particle-size distribution and a porous structure. According to the obtained results, the presented morphology could affect the release behavior of olive oil and probiotic bacteria.
3.8 Stability of the probiotics inside microparticles against in vitro stimulated gastrointestinal solutions
The results obtained by counting colonies after exposure to the simulated GI conditions are shown in Fig. 5A. The abilities of the two designated microparticles compared with un-coated cells to persist in the stomach solution (SGS) were different. The un-coated cells showed about 2.4 log reductions after 2 h of exposure, compared to the microcapsule samples, which reduced viability by about 0.85 and 0.93 log cycles for M1 and M2 microparticles, respectively. In detail, the population of microparticle samples after exposure to stomach conditions remained stable in the same log cycles without significant differences between them (p > 0.05). Also, the presence of WPC with SA as a coating material enhanced the stability of SA in acidic conditions. While after exposure to intestinal conditions (SIS), a decline in the viable counts was observed for un-coated cells, which reduced viability by about 5.6 log cycles after 6h.
On the other hand, both microparticle samples showed little reduction after the treatment in SIS at 6 h. Indeed, the count has reduced by about 2.87 and 2.27 log cycles at 6 h of exposure for M1 and M2, respectively. As previously stated, microencapsulated probiotics with M1 or M2 have the same behavior to maintain probiotic survival rate, but nanoclay with biopolymers SA-WPC materials in nanocomposites was found to be slightly superior to the viability of entrapped cells than nanoemulsion. The survivability rate after exposure to GI solutions for 6 h was recorded at 40.42, 70.00, and 77.00% for un-coated cells and M1 and M2 microparticles, respectively. Also, the presence of fig extract has a positive antioxidant effect on the probiotic cells, as mentioned before by El-Sayed et al. [13]. Generally, the encapsulated cells showed better viability once exposed to the stimulated GI conditions than un-coated cells. Our findings are in agreement with those of other previous works, where microencapsulation in using different biopolymers, as coating materials was found to enhance the survival of probiotics in GI conditions [10, 18, 28, 64]. Thus, microencapsulation could enhance the number of potential probiotics for food supplementation purposes.
3.9 Release rate of extra virgin olive oil and probiotics
The release pattern of EVOO from microparticles in vitro was evaluated in simulated digestion to examine the release behavior during gastrointestinal transit. In vitro, simulated digestion is generally divided into two divisions: simulated digestion in the stomach and simulated digestion in the intestine (Fig. 5B). In general, food was held for 2 h after entering the stomach and then went into the small intestine for more digestion for about 5 h [65]. As shown in Fig. 5B, M2 microparticles exhibited a slower release rate than M1 microparticles without nanoclay addition. In the first 2 h of the gastric solution, the microparticles were in the acidic media of gastric acid as intact at only <20%. The coating materials of M1 and M2 microparticles had a controlling effect on the release of gastric acid.
Although SA was not stable in acidic conditions, mixing it with WPC gave good protection to the olive oil entrapment. Borreani et al. [66] stated that the strong interaction between the oppositely charged protein and alginate produced slowed down protein degradation, and the aggregates formed between whey protein and alginate. Using whey protein as a coating material protected the entrapped substances such as linseed oil [67] and fish oil [68]. When digested in the small intestine for 2–6 h, the EVOO was released in large quantities from M1 microparticles due to the decomposition of trypsin and the acid-alkaline environment. At the end of 6 h, the core oil release rate of M2 microparticles is about 61% slower than M1 microparticles. This slow-release rate may be interpreted based on the good barrier properties of nanoclay [56]. While the total release from M1 microparticles was more than 75% of the encapsulated core oil, Zhang et al. [69] showed that the release profile of imidacloprid was retarded after incorporating it into bentonite–alginate composites.
The probiotic cells released from the prepared microparticles in simulated colonic solution at different times are shown in Fig. 5C. The release of probiotic cells from the microparticles and their colonization and growth in the colon are important. According to our findings, un-coated cells released 5.80 log CFU/g during the first 1 h of incubation in simulated colon solution. But little counts were released from different microparticles, which were around 3.30 and 3.00 log CFU/g for M1 and M2 microparticles, respectively. During the incubation time, the release rate was increased from all microparticles to reach the maximum level of 8.00 and 7.49 log CFU/g at 4 h for M1 and M2 microparticles, respectively. Also, the nanoclay with SA-WPC biopolymers in M2 has delayed the release rate in comparison with M1. According to the author Li et al. [41], this funding is the result of aggregated and adhered probiotic cells that are entrapped in M2 microparticles on the surface of nanoclay, which may delay cell release.
3.10 Production of functional yogurt
3.10.1 Microbiological evaluation
Differences in viable counts of Bifidobacterium in yogurt treatments during storage are presented in Fig. 6. Viable counts of Bifidobacterium were significantly enhanced during storage time for all treatments, but the most increased counts were detected at 15 days of storage, especially in treatments that were fortified with M1 and M2 microparticles. Generally, the Bifidobacterium counts in treatments with microparticles (T2M1 and T3M2) and the viable counts were located in log cycle 8, dissimilar to the treatment with un-coated cells (T1Cells), in which the viable counts were located in log cycle 6 at the end of storage. This increase shows the protective effect of encapsulation on the survival rate of Bifidobacterium.
The same results were observed for the counts of Lactobacilli (counts including L. rhamnosus and L. bulgaricus) using an MRS agar medium. Commonly, the most viable counts were recorded in treatments with microparticles (T2M1 and T3M2) than in treatments with un-coated cells (T1Cells). During the storage period, the counts of Lactobacilli were improved and the maximum viable counts were established at 10 days, followed by a slight decline for all treatments. Moreover, the greatest decline in viable counts was recorded for un-coated cells (T1Cells), where the viable counts could be affected by the acidity that developed during storage from the metabolic activity of starter culture and probiotics. From Fig. 6, the counts of Lactobacilli found in treatments with microparticles (T2M1 and T3M2) were located in log cycle 8, but in un-coated cells treatment (T1Cells), the Lactobacilli were located in Log cycle 7 at the end of the storage period.
Also, the counts of Streptococci as yogurt starter culture (S. thermophilus) were determined during storage in all treatments (Fig. 6). In the fresh samples, the streptococci counts were near in all treatments, which were located at log cycle 7. The maximum Streptococci counts were recorded at 10 days and followed by a count decline. T1Cell declines were greater in un-coated cells, which had a more acidic development, than in other microparticles treatments.
The counts of mold and yeast appeared first in treatments containing un-coated cells (T1Cells), in which little mold and yeast counts were recorded (1.90 log CFU/mL), and these counts increased with storage period to reach 3.98 log CFU/mL (Fig. 6). During the treatment with microparticle probiotics, the mold and yeast counts were recorded at 15 days of storage at about 1.39 and 1.33 log CFU/mL for T2M1 and T3M2, respectively. These findings could be related to the acidity production that encourages the growth of mold and yeast, especially in un-coated cells. Generally, the microparticle techniques have delayed the release of metabolic activity produced from the coated strains more than un-coated. These results were in agreement with those obtained by Jayalalitha et al. [70], and Fayed et al. [30] showed also that the encapsulated probiotic count in yogurt was significantly superior to that in the free cells every week of the storage period.
3.11 Antioxidant activity
The DPPH assay is based on the electronic change between the free radical and an antioxidant substance (Fig. 7). The purple color of DPPH is changed to a pale yellow when it is exposed to antioxidants. The antioxidant activity (AA) results of raw materials and prepared microparticles are represented in Fig. 7. It was noted that Fig extract showed a good power of antioxidants, followed by probiotic bacteria and olive oil. Elvan et al. [71] showed that probiotics scavenged 71.6% of DPPH activity. A range of activity between 78 and 65.8% was determined for M1 and M2 microparticles, respectively. The yogurt enriched with M1 showed better antioxidant activity than yogurt containing M2 microparticles. These results were in line with the oxidative stability results. Therefore, the M1 microparticles have a better OS and AA than the M2 microparticles. It is very interesting to observe the enhancement in antioxidant activity after the microencapsulation process and after adding the powder to yogurt.
3.12 Chemical properties
The chemical composition of yogurt treatments is found in Table 5. The total solid (TS) ranged between 16.08 and 17.88%, and the high total solid content was indicated in T2M1 and T3M2, which related to the coated materials of SA-WP. Additionally, the moisture content was different in all treatments, and little moisture content was found in T2M1 and T3M2. Generally, the moisture content ranged between 82.28 and 83.13. The change in the total solid and moisture content in the T2M1 and T3M2 that are integrated with microparticles may be related to the found coating materials (SA-WPC). Furthermore, there were minor but non-significant differences in total protein, which ranged between 3.85 and 4.03%. Also, this slight increase in protein content was recorded for microencapsulated treatments. The pH values in the treatments ranged between 4.20 and 4.75, but more of a drop in the pH value was observed in the T1Cells that integrated un-coated cells. The development in the pH was fast for T1cells and was related to the metabolic activities of free un-coated cells. In the same line, the titratable acidity (TA) ranged between 0.97 and 1.20, and the higher acidity value for T1cells was followed by T2M1 and T3M2, which found coating materials were slow in acidity release. Our results are in line with Ismail et al. [64] and Fayed et al. [30], which indicated that the pH values were lower in yogurt with free cells than in yogurt with encapsulated probiotics.
3.13 Sensory evaluation
Sensory evaluations of yogurt treatments during storage are shown in Table 6. Fresh treatments were given the same scores for flavor, body, and texture, and slight differences were observed for appearance, which this difference related to the color of treatments fortified with microparticles. In T2M1 and T3M2, the color of yogurt changes in color more than T1Cells, which found microparticles containing fig leaf extract with EVOO, giving the yogurt color near green. During storage, the appearance score decreased with time, and this decrease was related to the formation of syneresis and a change in the color of yogurt, especially in treatment containing microparticles.
On the other hand, the flavor in treatments that contained microparticles gained a higher score than T1Cells that contained un-coated cells. This funding was related to developing a more acidic flavor in T1Cells than other treatments and improving moldy flavor resulting from higher mold and yeast counts in T1Cells than other treatments [72]. Also, body and texture decreased with the storage period for all treatments, with T1Cells showing the greatest decline in the score, which was related to the formation of more syneresis with storage [73]. Generally, the treatments fortified with microparticles, either M1 or M2, gained a near score in sensory evaluation without significant differences and had more scores than T1Cells that were fortified with un-coated cells. But the yogurt with microparticles gained a low score in appearance that T1Cells related to the change in color of the resulting yogurt. In our opinion, it is possible to avoid the change of color by fortifying the yogurt with fruits of green color to make it more acceptable to the consumer. Our findings suggest that functional yogurt can be used as a new product to deliver vital components and probiotic strains.
4 Conclusion
Using ultra-sonication and freeze-drying techniques, functional nanoemulsion and nanocomposite microparticles were produced. It was noted that nanocomposite demonstrated a larger size and a slight increase in stability than nanoemulsion using the DLS technique. Therefore, the oxidative stability of extracted oil from M1 microparticles was higher than that of M2 microparticles. In contrast, the M2 microparticles showed the highest encapsulation efficiency and slow-release rate for extracted EVOO (61%) and probiotics (7.49 log CFU/g). These microparticles showed slightly superior viability of entrapped cells than the M1 microparticles. However, the inhibition zones were in M1 (11 to 20 mm) more than M2 (9 to 18 mm) microparticles using a different strain of microorganisms. M2 microparticles revealed the greatest fold change versus M1 microparticles in Caco-2 and HCT 116 cells. Overall, this study recommended that EVOO be used not only as a flavoring and cooking fat but also as an ingredient in a broad range of food products. Thus, the microencapsulation technique might be seen as a useful tool for the addition of EVOO and probiotics to functional foods. As well, the addition of these microparticles to yogurt may be interesting to sensitive cancer patients due to its anticolorectal cancer properties.
Data availability
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
References
Calvo P, Castaño ÁL, Lozano M, González-Gómez D (2012) Influence of the microencapsulation on the quality parameters and shelf-life of extra-virgin olive oil encapsulated in the presence of BHT and different capsule wall components. Food Res Int 45(1):256–261
Jimenez-Lopez C, Carpena M, Lourenço-Lopes C, Gallardo-Gomez M, Lorenzo JM, Barba FJ et al (2020) Bioactive compounds and quality of extra virgin olive oil. Foods 9(8):1014
Gargouri B, Zribi A, Bouaziz M (2015) Effect of containers on the quality of Chemlali olive oil during storage. J Food Sci Technol 52(4):1948–1959
Nie P, Li Z, Wang Y, Zhang Y, Zhao M, Luo J et al (2019) Gut microbiome interventions in human health and diseases. Med Res Rev 39(6):2286–2313
Baek YJ, Lee BH (2009) Probiotics and prebiotics as bioactive components in dairy products. Bioactive Componentsin Milk and Dairy Products, 287
Divya JB, Varsha KK, Nampoothiri KM, Ismail B, Pandey A (2012) Probiotic fermented foods for health benefits. Eng Life Sci 12:377–390
Liu X, Cao S, Zhang X (2015) Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J Agric Food Chem 63(36):7885–7895
Quigley EM (2019) Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol 17(2):333–344
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV (2012) Microencapsulation of probiotics for gastrointestinal delivery. J Control Release 162(1):56–67
El-Sayed SM, Elaaser M, El-Sayed HS (2021a) Ameliorate the processed cheese production by functional microparticles loaded with mustard seed extract and Bifidobacterium bifidum. Biocatalys Agric Biotech 38:102221
Hamed SF, Hashim AF, Abdel Hay H, Abd-Elsalam KA, El-Sherbiny IM (2022) Microencapsulation of omega-3 rich flaxseed and fish oils. Egypt J Chem 65(2):623–638
Zhang Y, Wan Y, Huo B, Li B, Jin Y, Hu X (2018a) Extracts and components of Ficus carica leaves suppress survival, cell cycle, and migration of triple-negative breast cancer MDA-MB-231 cells. OncoTargets Ther 11:4377
El-Sayed S, El-Sayed H, Salama HH, Abd-Rabou N (2021b) Preparation and evaluation of microencapsulated fig leaves extract for production novel processed cheese sauce. Egypt J Chem 64(4):1665–1678
Fahimdanesh M, Tafazolifard V, Afzali E (2015) The effect of adding fig leaf extracts by thermal method on the physicochemical properties and oxidative stability of virgin olive oil. International Journal of Food Engineering, 1(2)
Tee WF, Nazaruddin R, Tan YN, Ayob MK (2014) Effects of encapsulation on the viability of potential probiotic Lactobacillus plantarum exposed to high acidity condition and presence of bile salts. Food Sci Technol Int 20(6):399–404
Al-Furaih LY, Ababutain IM, Abd-El-Khalek AB, Abdel-Salam AM (2016) Effect of different microencapsulation materials on stability of Lactobacillus plantarum DSM 20174. Afr J Biotechnol 15(24):1207–1216
Salahuddin N, Elbarbary A, Allam NG, Hashim AF (2018) 5-Phenyl-1,3,4-oxadiazole-2-thiol/polyamide-montmorillonitemicrobicidesnanocomposites as drug delivery system. J Phys Org Chem 31(7):e3834
Arjona JC, Silva-Valenzuela MG, Wang SH, Valenzuela-Diaz FR (2021) Biodegradable nanocomposite microcapsules for controlled release of urea. Polymers 13:722
Hamed SF, Hashim AF, Hamid HAA, Abd-Elsalam KA, Golonka I, Musiał W, El-Sherbiny IM (2020) Edible alginate/chitosan-based nanocomposite microspheres as delivery vehicles of omega-3 rich oils. Carbohydr Polym 239:116201
Silva KA, Coelho MAZ, Calado VM, Rocha-Leão MH (2013) Olive oil and lemon salad dressing microencapsulated by freeze-drying. LWT-Food Sci Technol 50(2):569–574
Kowalska E, Ziarno M, Ekielski A, Żelaziński T (2022) Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules 27(10):3321
El-Sayed HS, Youssef K, Hashim AF (2022) Stirred yogurt as a delivery matrix for freeze-dried microcapsules of synbiotic EVOO nanoemulsion and nanocomposite. Front Microbiol 13:893053
Fayed B, Abood A, El-Sayed HS, Hashem AM, Mehanna NSH (2018) A synbiotic multiparticulate microcapsule for enhancing inulin intestinal release and Bifidobacterium gastro-intestinal survivability. Carbohydr Polym 193:137–143
El-Sayed SM, El-Sayed HS (2021) Antimicrobial nanoemulsion formulation based on thyme (Thymus vulgaris) essential oil for UF labneh preservation. J Mater Res Technol 10:1029–1041
Meerloo JV, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. In Cancer cell culture (pp. 237-245). Humana Press
Carneiro HC, Tonon RV, Grosso CR, Hubinger MD (2013) Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J Food Eng 115(4):443–451
Jafari SM, Assadpoor E, He Y, Bhandari B (2008) Encapsulation efficiency of food flavours and oils during spray drying. Dry Technol 26(7):816–835
Mahmoud M, Abdallah NA, El-Shafei K, Tawfik NF, El-Sayed HS (2020) Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon 6(3):e03541
Lotfipour F, Mirzaeei S, Maghsoodi M (2012) Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci World J 2012:1–8
Fayed B, El-Sayed HS, Abood A, Hashem AM, Mehanna NS (2019) The application of multi-particulate microcapsule containing probiotic bacteria and inulin nanoparticles in enhancing the probiotic survivability in yoghurt. Biocatalys Agric Biotech 22:101391
IDF, International Dairy Federation (1997) Dairy starter cultures of lactic acid bacteria (LAB) standard of identity, IDF Standard No. 149A. Brussels
APHA (1994) Standard methods for examination of dairy products (16thed.). American Public. Health Association, Washington, DC
Ling ER (1963) A text book of dairy chemistry, vol.2, practical (3rded.). London: Chapman and Hall Ltd58–65
AOAC (2012) Methods of analysis. Vol.1. Agricultural chemicals, contaminants, drugs.19th ed. Washington DC
Davis JG (1965) Cheese. Vol. I. Basic technology. Cheese. Vol. I. Basic technology
SAS (2004) Statistical analyses systems. SAS, User guide statistics. SAS. Institute Inc., Cary
Peng F, Ke Y, Zhao Y, Hu X, Zhao X (2019) The influence of organically intercalated montmorillonites on the interfacial tension and structure of oil-in-water nanoemulsions. RSC Adv 9(24):13378–13385
Nciri H, Benna-Zayani M, Stambouli M, Kbir-Ariguib N, Trabelsi-Ayadi M, Rosilio V, Grossiord JL (2009) Influence of clay addition on the properties of olive oil in water emulsions. Appl Clay Sci 43(3-4):383–391
Sulaiman ISC, Basri M, Masoumi HRF, Ashari SE, Ismail M (2016) Design and development of a nanoemulsion system containing extract of Clinacanthus nutans (L.) leaves for transdermal delivery system by D-optimal mixture design and evaluation of its physicochemical properties. RSC Adv 6(71):67378–67388
Barradas TN, de Campos VEB, Senna JP, Coutinho CDSC, Tebaldi BS, Silva KGDH, Mansur CRE (2015) Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloids Surf A Physicochem Eng Asp 480:214–221
Li S, Jiang C, Chen X, Wang H, Lin J (2014) Lactobacillus casei immobilized onto montmorillonite: survivability in simulated gastrointestinal conditions, refrigeration and yogurt. Food Res Int 64:822–830
Rashid KI, Mahdi NM, Alwan MA, Khalid LB (2014) Antimicrobial activity of fig (Ficus carica Linn.) leaf extract as compared with latex extract against selected bacteria and fungi. J Babylon Univ/Pure Appl Sci 5(22):1620–1626
Saleh B, Hammoud R, Al-Mariri A (2015) Antimicrobial activity of Ficus sycomorus L. (Moraceae) leaf and stem-bark extracts against multidrug resistant human pathogens. HerbaPolonica 61(1):39–49
Hussein SA (2013) Antimicrobial activity of probiotic bacteria. Egyptian Academic J Biol Sci, G Microbiol 5(2):21–34
Ambalam P, Kondepudi KK, Balusupati P, Nilsson I, Wadström T, Ljungh A (2015) Prebiotic preferences of human lactobacilli strains in co-culture with bifidobacteria and antimicrobial activity against Clostridium difficile. J Appl Microbiol 119(6):1672–1682
Erginkaya Z, Konuray G, Harmanci M, Kesen G, Nagihan METE (2019) Antibacterial effects of microencapsulated probiotic and synbiotics. Çukurova Tarımve Gıda Bilimleri Dergisi 34(1):27–36
Rostam MA, Salleh MS, Harun AF, Ali AN (2018) The antimicrobial activities of fig (Ficus carica L.) leaves extract against Staphylococcus aureus and Escherechia coli. Int J Allied Health Sci 2(1):273–284
Mahmoudi S, Khali M, Benkhaled A, Benamirouche K, Baiti I (2016) Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac J Trop Biomed 6(3):239–245
Denkova R, Goranov B, Teneva D, Denkova Z, Kostov G (2017) Antimicrobial activity of probiotic microorganisms: mechanisms of interaction and methods of examination. Antimicrobial Res: Novel bioknowledge Educ Prog 1:201–212
Abduljauwad SN, Ahmed HUR (2019) Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci Rep 9(1):1–12
Grimes WR, Luo Y, McFarland AW, Mills DK (2018) Bi-functionalized clay nanotubes for anti-cancer therapy. Appl Sci 8(2):281
Mahkam M, Zeynabad FB, Alizadeh E, Rahimi M, Rahimi F, Salehi R (2021) Novel methotrexate-ciprofloxacin loaded alginate-clay based nanocomposite as anticancer and antibacterial co-drug delivery system. Advanced Pharmaceut Bull 11(3):477
Abd-Rabou AA, Edris AE (2021) Cytotoxic, apoptotic, and genetic evaluations of Nigella sativa essential oil nanoemulsion against human hepatocellular carcinoma cell lines. Cancer Nanotechnol 12(1):1–23
Bharmoria P, Bisht M, Gomes MC, Martins M, Neves MC, Mano JF et al (2021) Protein-olive oil-in-water nanoemulsions as encapsulation materials for curcumin acting as anticancer agent towards MDA-MB-231 cells. Sci Rep 11(1):1–12
Pampaloni B, Mavilia C, Fabbri S, Romani A, Ieri F, Tanini A et al (2014) In vitro effects of extracts of extra virgin olive oil on human colon cancer cells. Nutr Cancer 66(7):1228–1236
Singha S, Hedenqvist MS (2020) A review on barrier properties of poly(lactic acid)/clay nanocomposites. Polymers 12(5):1095
Koç M, Güngör Ö, Zungur A, Yalçın B, Selek I, Ertekin FK, Ötles S (2015) Microencapsulation of extra virgin olive oil by spray drying: effect of wall materials composition, process conditions, and emulsification method. Food Bioprocess Technol 8:301–318
Zhang XP, Luo J, Zhang DX, Jing TF, Li BX, Liu F (2018b) Porous microparticles with tunable pore sizes provide easily controllable release and bioactivity. J Colloid Interface Sci 517:86–92
Jyothi NVN, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan GY (2010) Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul 27(3):187–197
Schlicher EJ, Postma NS, Zuidema J, Talsma H, Hennink WE (1997) Preparation and characterisation of poly(D,L-lactic-co-glycolic acid) microspheres containing desferrioxamine. Int J Pharm 153(2):235–245
Allaoui S, Bennani MN, Ziyat H, Qabaqous O, Tijani N, Ittobane N, …, Filali FR (2020) Antioxidant and antimicrobial activity of polyphenols extracted after adsorption onto natural clay “Ghassoul”. J Chem, 8736721
El Yamani M, Sakar EH, Mansouri F, Serghini-Caid H, Elamrani A, Rharrabti Y (2019) Effect of pigments and total phenols on oxidative stability of monovarietal virgin olive oil produced in Morocco. RivItalSostanze Gr 96:17–24
Hao F, Fu N, Ndiaye H, Woo MW, Jeantet R, Chen XD (2021) Thermotolerance, survival, and stability of lactic acid bacteria after spray drying as affected by the increase of growth temperature. Food Bioprocess Technol 14(1):120–132
Ismail SA, El-Sayed HS, Fayed B (2020) Production of prebiotic chitooligosaccharide and its nano/microencapsulation for the production of functional yoghurt. Carbohydr Polym 234:115941
Sensory, I. (2021).A review on the food digestion in the digestive tract and the used in vitro models. Current Res Food Sci, 4, 308-319.
Borreani J, Llorca E, Larrea V, Hernando I (2016) Adding neutral or anionic hydrocolloids to dairy proteins under in vitro gastric digestion conditions. Food Hydrocoll 57:169–177
Gallardo G, Guida L, Martinez V, López MC, Bernhardt D, Blasco R, Pedroza-Islas R, Hermida LG (2013) Microencapsulation of linseed oil by spray drying for functional food application. Food Res Int 52:473–482
Aghbashlo M, Mobil H, Madadlou A, Rafiee S (2013) Influence of wall material and inlet drying air temperature on the microencapsulation of fish oil by spray drying. Food Bioprocess Technol 6:1561–1569
Zhang H, Shi Y, Xu X, Zhang M, Ma L (2020) Structure regulation of bentonite-alginate nanocomposites for controlled release of imidacloprid. ACS omega 5(17):10068–10076
Jayalalitha V, PalaniDorai R, Dhanalakshmi B, Elango A, Nasresh KC (2011) Improving the viability of probiotics in yoghurt through different methods of encapsulation. Egyptian J Dairy Sci 39:39–44
Elvan M, Baysal AH, Harsa S (2022) Microencapsulation of a potential probiotic Lactiplantibacillus pentosus and its impregnation onto table olives. LWT 156:112975
Ribeiro MCE, Chaves KS, Gebara C, Infante FN, Grosso CR, Gigante ML (2014) Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res Int 66:424–431
Temesgen M, Yetneberk S (2015) Effect of application of stabilizers on gelation and synersis in yoghurt. Food Sci Qual Manag 37:90–102
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to that manuscript regarding design of experiments, experimental work, preparing and reviewing the manuscript before submission.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashim, A.F., Abd-Rabou, A.A. & El-Sayed, H.S. Functional nanoemulsion and nanocomposite microparticles as an anticolorectal cancer and antimicrobial agent: applied in yogurt. Biomass Conv. Bioref. 14, 13233–13249 (2024). https://doi.org/10.1007/s13399-022-03313-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03313-3