Abstract
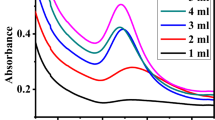
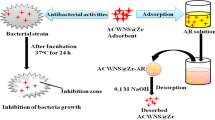
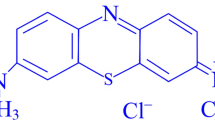
In this present work, microwave-mediated physical structural modification (PSM) of water hyacinth biomass and its impact on methylene blue biosorption kinetics and gold nanoparticle synthesis potential were studied. Microwaves with the strength of 750 W for 60 min brought about a notable impact on the morphology of the biomass by the formation of pores followed by enhancement of cellulose hemicellulose with significant lignin reduction. Batch adsorption studies were conducted under in vitro conditions using various isotherms such as Langmuir plot, Freundlich and Dubinin mechanism. The biosorption capacity for monolayer formation was maximum with a value of 2.45 × 10−3 mol g−1 at a process temperature of 30 °C. The intensity of biosorption that changed from 21.65 to 9.54 showed that the biosorption process was favourable due to the formation of n value in the range of 1–10. RL [RL = 1 / (1 + KLCo)] which confirmed the favourable process of biosorption. Obtained results indicated that the pseudo-second-order model better described the biosorption experimental data. Highly stable nanodimensional gold nanoparticles with an antibacterial action against waterborne pathogenic bacterial strains Escherichia coli and Enterococcus faecalis were synthesised from PSM biomass extract. Biocompatibility of the synthesized gold nanoparticles that tested on Vero cell line revealed no specific impact on cell viability, antioxidative enzyme status and LDH release. These findings suggested the possible utilisation of PSM biomass as an effective adsorbent for toxic pollutants and, hence, a hotspot for synthesising active antibacterial gold nanoparticles.













Similar content being viewed by others
Data availability (data transparency)
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability (software application or custom code)
Not applicable to this study.
Abbreviations
- g:
-
Gram
- °C:
-
Degree Centigrade
- OD:
-
Optical density
- LDH:
-
Lactate dehydrogenase
- MTT:
-
Dimethyl thiazol tetrazolium component
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- GST:
-
Glutathione S transferase
- EDAX:
-
Energy-dispersive atomic beam spectroscopy
- PSM:
-
Physically structured and modified
- WHB:
-
Water hyacinth biomass
References
Mousavi SA, Mehralian M, Khashij M, Parvaneh S (2017) Methylene blue removal from aqueous solutions by activated carbon prepared from N. Microphyllum (AC-NM): RSM analysis, isotherms and kinetic studies. Glob Nest J 19:697–705. https://doi.org/10.30955/gnj.002422
Arshad R, Bokhari TH, Javed T, Bhatti IA, Rasheed S, Iqbal M,... & Zia-ur-Rehman M (2020) Degradation product distribution of Reactive Red-147 dye treated by UV/H2O2/TiO2 advanced oxidation process. J Mater Res Tech 9(3), 3168–3178
Valli Nachiyar C, Namasivayam SKR, Kumar RR, Sowjanya M (2014) Bioremediation of textile effluent containing Mordant Black 17 by bacterial consortium CN-1. J Water Process Eng 4:196–200. https://doi.org/10.1016/j.jwpe.2014.10.003
Rahmat M, Rehman A, Rahmat S, Bhatti HN, Iqbal M, Khan WS,... & Nazir A (2019) Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J Mater Res Tech 8(6), 5149–5159
Kausar A, Naeem K, Hussain T, Bhatti H N, Jubeen, F, Nazir A, Iqbal M (2019) Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: comparison of linear and non-linear regression methods. J Mat Res Tech 8(1):1161–1174
Bhatti HN, Safa Y, Yakout SM, Shair OH, Iqbal M, Nazir A (2020) Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: adsorption/desorption, kinetics and recycling studies. Int J Bio Macromol 150:861–870
Bhatti HN, Iqbal M, Nazir A, Ain H (2020) Kinetic study of degradation of basic turquise blue X-GB and basic blue X-GRRL using advanced oxidation process. Z Phys Chem 234(11–12):1803–1817
Bukhari A, Atta M, Nazir A, Shahab MR, Kanwal Q, Iqbal M, ... & Alwadai N (2022) Catalytic degradation of MO and MB dyes under solar and UV light irradiation using ZnO fabricated using Syzygium Cumini leaf extract. Zeitschrift für Physikalische Chemie, 236(5), 659–671
Elgarahy AM, Elwakeel KZ, Mohammad SH, Elshoubaky GA (2021) A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Cleaner Engg Tech 4:100209
Elwakeel KZ (2010) Environmental application of chitosan resins for the treatment of water and wastewater: a review. J Disper Sci Tech 31(3):273–288
Elwakeel KZ, Elgarahy AM, Khan ZA, Almughamisi MS, Al-Bogami AS (2020) Perspectives regarding metal/mineral-incorporating materials for water purification: with special focus on Cr (vi) removal. Mater Adv 1(6):1546–1574
Elgarahy AM, Elwakeel KZ, Elshoubaky GA, Mohammad SH (2019) Untapped sepia shell–based composite for the sorption of cationic and anionic dyes. Water Air Soil Pollu 230(9):1–23
Erkan HS, Engin GO (2017) The investigation of paper mill industry wastewater treatment and activated sludge properties in a submerged membrane bioreactor. Water Sci Technol 76(7):1715–1725
Elwakeel KZ, Yousif AM (2010) Adsorption of malathion on thermally treated egg shell material. Water Sci Technol 61(4):1035–1041
Mashabi RA, Khan ZA, Elwakeel KZ(2022) Chitosan- or glycidyl methacrylate-based adsorbents for the removal of dyes from aqueous solutions: a review. Mater Adv 3:5645–5671. https://doi.org/10.1039/d2ma00320
Erkan HS, Engin GO (2017) The investigation of paper mill industry wastewater treatment and activated sludge properties in a submerged membrane bioreactor. Water Sci Technol 76:1715–1725
Bhattacharjee C, Dutta S, Saxena VK (2020) A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ Adv 2:100007
Hussein KO, Pandey S, Ogunkunle CO, Ngila CJ, Zvinowanda C, Jimoh I, Lawal IA, Orosun MM, Adeniy AG (2022) Nanomaterial-based biosorbents: adsorbent for efficient removal of selected organic pollutants from industrial wastewater. Emerg Contaminants 8:46–58
Heo YJ, Park SJ (2017) Facile synthesis of MgO-modified carbon adsorbents with microwave-assisted methods: effect of MgO particles and porosities on CO2 capture. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-06091-5
Jamil A, Bokhari TH, Javed T, Mustafa R, Sajid M, Noreen S, ..., Jilani MI (2020) Photocatalytic degradation of disperse dye Violet-26 using TiO2 and ZnO nanomaterials and process variable optimization. J Mater Res Tech 9(1):1119–1128
Kaur S, Rani S, Mahajan RK (2015) Adsorption of dye crystal violet onto surface-modified Eichhornia crassipes. Desalin Water Treat 53:1957–1969. https://doi.org/10.1080/19443994.2013.859104
Fahmi AH, Samsuri AW, Jol H, Singh D (2018) Physical modification of biochar to expose the inner pores and their functional groups to enhance lead adsorption. RSC Adv 8:38270–38280. https://doi.org/10.1039/c8ra06867d
Ge X, Wu Z, Wu Z, Yan Y, Cravotto G, Ye BC (2016) Microwave-assisted modification of activated carbon with ammonia for efficient pyrene adsorption. J Ind Eng Chem 39:27–36. https://doi.org/10.1016/j.jiec.2016.05.003
Inyinbor AA, Adekola FA, Olatunji GA, (2020) Microwave-assisted urea modified crop residue in Cu2+ scavenging. Heliyon 6:e03759. https://doi.org/10.1016/j.heliyon.2020.e03759
Jones JL, Jenkins RO, Haris PI (2018) Extending the geographic reach of the water hyacinth plant in removal of heavy metals from a temperate Northern Hemisphere river. Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-29387-6
Elwakeel KZ, Elgarahy AM, Guibal E (2021) A biogenic tunable sorbent produced from upcycling of aquatic biota-based materials functionalized with methylene blue dye for the removal of chromium(VI) ions. J Environ Chem Eng 9:104767.
Liu P, Wu Z, Ge X, Yang X (2019) Hydrothermal synthesis and microwave-assisted activation of starch-derived carbons as an effective adsorbent for naphthalene removal. RSC Adv 9:11696–11706. https://doi.org/10.1039/c9ra01386e
Liu Z, Zhou X, Wu F, Liu Z (2020) Microwave-assisted preparation of activated carbon modified by zinc chloride as a packing material for column separation of saccharides. ACS Omega 5:10106–10114. https://doi.org/10.1021/acsomega.0c00674
Mashabi RA, Khan ZA, Elwakeel KZ (2022) Chitosan or glycidyl methacrylate-based adsorbents for dyes removal from the aqueous solutions: a brief review. Mater Adv 1:1–27
Akendo Innocent CO, Gumbe Lawrence O, Gitau Ayub N (2008) Dewatering and drying characteristics of water hyacinth (Eichhornia Crassipes) petiole. Part II. Drying characteristics Agricultural Engineering International: the CIGR Ejournal Manuscript FP 07033.10:1–11
Nisar N, Ali O, Islam A, Ahmad A, Yameen M, Ghaffar A, ... & Masood N (2019) A novel approach for modification of biosorbent by silane functionalization and its industrial application for single and multi-component solute system. Zeitschrift für Physikalische Chemie 233(11), 1603–1623
Nazir A, Zahra F, Sabri MU, Ghaffar A, Ather AQ, Khan MI, Iqbal M (2021) Charcoal prepared from bougainvillea spectabilis leaves as low cost adsorbent: kinetic and equilibrium studies for removal of iron from aqueous solution. Z Phys Chem 235(3):265–279
Okoro HK, Pandey S, Ogunkunle CO, Ngila CJ, Zvinowanda C, Jimoh I, ... & Adeniyi AG (2022) Nanomaterial-based biosorbents: adsorbent for efficient removal of selected organic pollutants from industrial wastewater. Emerg Contaminants 8, 46–58
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Kumar JA, Krithiga T, Anand KV, Sathish S, Namasivayam SKR, Renita AA, Bandegharaei AH, Praveenkumar TR, Rajasimman M, Bhat NS, Dutta S (2021) Kinetics and regression analysis of phenanthrene adsorption on the nanocomposite of CaO and activated carbon: characterization, regeneration, and mechanistic approach. J Mol Liqu 334:116080
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–152
Kumar JA, Amarnath DJ, Jabasingh SA, Kumar PS, Anand KV, Narendrakumar G, Namasivayam SKR, Krithiga T, Sunny S, Pushkala SP, Yuvarajan D (2019) One pot green synthesis of nano magnesium oxide-carbon composite: preparation, characterization and application towards anthracene adsorption. J Clean Prod 237:116791
Kumar JA, Kumar PS, Krithiga T, Prabu D, Amarnath DJ, Sathish S, Venkatesan D, Bandegharaei AH, Prashanth P (2021) Acenaphthene adsorption onto ultrasonic assisted fatty acid mediated porous activated carbon-characterization, isotherm and kinetic studies. Chemosphere 284:131249
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195. https://doi.org/10.1016/0003-9861(78)90479-4
Praveen Kumar R, Varanasi S, Purushothaman V (2010) Investigation of the biosorption mechanisms of methylene blue onto press mud through kinetic modelling analysis. Indian J Sci Technol 3:44–47. https://doi.org/10.17485/ijst/2010/v3i1/29642
Rekha D, Anajali S (2014) Biosorption of methylene blue from aqueous solution onto green seaweeds. Int J of Rec Sci Res 5:347–351
Saif Ur Rehman M, Han JI (2013) Biosorption of methylene blue from aqueous solutions by Typha angustata phytomass. Int J Environ Sci Technol 10:865–870. https://doi.org/10.1007/s13762-012-0128-5
Yu J, Zhang X, Wang D, Li P (2018) Adsorption of methyl orange dye onto biochar adsorbent prepared from chicken manure. Water Sci Technol 77:1303–1312
Yaseen DA, Scholz M (2019) Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol.16:1193–1226. https://doi.org/10.1007/s13762-018-2130-z
Yel O (2018) Biosorption of methylene blue and acid red 88 from wastewater by using peanut shell. Int J Chem Technol 2:56–62. https://doi.org/10.32571/ijct.415771
Zhang L, Cui L, Wang Z, Dong Y (2016) Modification of activated carbon using microwave radiation and its effects on the adsorption of SO2. J Chem Eng Japan 49:52–59. https://doi.org/10.1252/jcej.14we081
Rahmat M, Rehman A, Rahmat S, Bhatti HN, Iqbal M, Khan WS, ... & Nazir A (2019) Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J Mater Res Tech 8(6), 5149–5159
Khasri A, Khan MNN, Ahmad MA (2019) Microwave-assisted KOH activation preparation of groundnut shell based activated carbon for methyl yellow adsorption, in: AIP Conference Proceedings. https://doi.org/10.1063/1.5117116
Vijayaraghavan K, Mao J, Yun YS (2008) Biosorption of methylene blue from aqueous solution using free and polysulfone-immobilized Corynebacterium glutamicum: batch and column studies. Bioresour Technol 99:2864–2871. https://doi.org/10.1016/j.biortech.2007.06.008
Yadav V, Ali J, Garg MC (2021) Biosorption of methylene blue dye from textile-industry wastewater onto sugarcane bagasse: response surface modeling, isotherms, kinetic and thermodynamic modeling. J Hazardous Toxic Radioact Waste 25:4020067. https://doi.org/10.1061/(asce)hz.2153-5515.0000572
Lavado-Meza C, Asencios YJO, Cisneros-Santos G, Unchupaico-Payano I (2021) Removal of methylene blue dye using Nostoc commune biomass: kinetic, equilibrium and thermodynamic study. Rev Mex Ing Quim 20:941–954. https://doi.org/10.24275/rmiq/IA2291
Teja DD, Bhavani M, Sridevi V, Snehalatha P, Lohita M (2013) Biosorption of methylene blue dye from aqueous solution using papaya peel. Int J Innov Res Sci Eng Technol 2:4073–4081
Salazar-Rabago JJ, Leyva-Ramos R, Rivera-Utrilla J, Ocampo-Perez R, Cerino-Cordova FJ (2017) Biosorption mechanism of methylene blue from aqueous solution onto white pine (Pinus durangensis) sawdust: effect of operating conditions. Sustain Environ Res 27:32–40. https://doi.org/10.1016/j.serj.2016.11.009
Acknowledgements
We acknowledge SAIF IIT Madras for analytical methods of gold nanoparticles.
Author information
Authors and Affiliations
Contributions
The authors equally contributed to this study.
Corresponding author
Ethics declarations
Ethics approval (include appropriate approvals or waivers)
Not applicable to this study.
Consent to participate (include appropriate statements)
Not applicable to this study.
Consent for publication (include appropriate statements)
Not applicable to this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Microwave treatment of water hyacinth biomass (PSM) brought about enhanced biosorption of methylene blue.
• Biosorption equilibrium revealed by the Langmuir and Freundlich isotherms.
• Highly stable, nanodimensional gold nanoparticles (AuNps) with high antibacterial active AuNps were synthesized.
• Biocompatibility of the synthesized nanoparticles that tested on Vero cell line indicates no sign of cytotoxicity.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Namasivayam, S.K.R., Srinivasan, S., Kavisri, M. et al. Methylene blue biosorption and antibacterial active gold nanoparticle synthesis using microwave-treated structurally modified water hyacinth biomass. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03216-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03216-3