Abstract
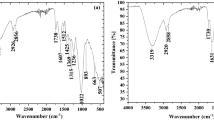
Agricultural wastes (AWs) are available abundantly at no or low costs; however, in most cases, not used reasonably. Despite their interesting chemical properties, coconut shells (CS), almond shells (AS), walnut shells (WS), and peanut shells (PS) are usually burned in the fields or discharged without any valorization. These AWs were investigated as low-cost bio-adsorbents to remove copper ions (Cu2+) from aqueous solutions. The four adsorbents were characterized using X-ray diffraction (XRD), the Fourier transform infrared spectra (FTIR), nitrogen adsorption/desorption measurements, scanning electron microscopy, and energy-dispersion X-ray spectroscopy (SEM–EDS). Characterization results revealed that the materials under investigation had porous surfaces, rich in fibers, and several potential adsorption sites. Therefore, their adsorption capacity for Cu2+ removal was evaluated under different operating conditions. Results showed that the CS had the best adsorption capacity among tested AWs. Under optimized parameters, the highest adsorption capacity was found 25, 18, 10, and 5 mg/g for WS, CS, PS, and AS, respectively. The adsorption of Cu2+ on the four adsorbents followed the second-order rate equation and the Langmuir adsorption isotherm model. After the adsorption process, the characterization of studied materials revealed no structural changes, proving the physical adsorption of Cu2+ on shells through long-range interactions between Cu2+ and reactive sites of adsorbents. The high adsorption capacity of the selected adsorbent was attributed to the presence of high content of cellulose compared to lignin.









Similar content being viewed by others
References
Wang F, Pan Y, Cai P et al (2017) Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent. Biores Technol 241:482–490
Periyasamy S, Kumar IA, Viswanathan N (2020) Activated carbon from different waste materials for the removal of toxic metals. In: Green materials for wastewater treatment. Springer, pp 47–68
Ihsanullah AA, Al-Amer AM et al (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161. https://doi.org/10.1016/j.seppur.2015.11.039
Varsha M, Senthil Kumar P, SenthilRathi B (2022) A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere 287:132270. https://doi.org/10.1016/j.chemosphere.2021.132270
Xu L, Suo H, Liu R (2021) Preparation and characterization of magnetic bioadsorbent for adsorp100111tion of Cd (II) ions. J Indian Chem Soc 98:100111
Salih SS, Ghosh TK (2017) Preparation and characterization of bioadsorbent beads for chromium and zinc ions adsorption. Cogent Environ Sci 3:1401577
Raut PN, Gotmare SR (2019) adsorption of Cu ions from wastewater by natural medicinal Bioadsorbent. Inter J Res App Sci & Eng Tech 7:876
He L, Yang C, Ding J et al (2022) Fe, N-doped carbonaceous catalyst activating periodate for micropollutant removal: significant role of electron transfer. Appl Catal B 303:120880. https://doi.org/10.1016/j.apcatb.2021.120880
Zhang L, Wang L, Zhang Y et al (2022) The performance of electrode ultrafiltration membrane bioreactor in treating cosmetics wastewater and its anti-fouling properties. Environ Res 206:112629. https://doi.org/10.1016/j.envres.2021.112629
Chakraborty R, Asthana A, Singh AK et al (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem 102:342–379. https://doi.org/10.1080/03067319.2020.1722811
Bilal M, Ihsanullah I, Younas M, Ul Hassan Shah M (2021) Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: a critical review. Sep Purif Technol 278:119510. https://doi.org/10.1016/j.seppur.2021.119510
Shi C, Wu Z, Yang F, Tang Y (2021) Janus particles with pH switchable properties for high-efficiency adsorption of PPCPs in water. Solid State Sci 119:106702. https://doi.org/10.1016/j.solidstatesciences.2021.106702
Liu W, Li J, Zheng J et al (2020) Different Pathways for Cr(III) Oxidation: implications for Cr(VI) Reoccurrence in Reduced Chromite Ore Processing Residue. Environ Sci Technol 54:11971–11979. https://doi.org/10.1021/acs.est.0c01855
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions — a review. Biores Technol 99:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Saheed IO, Oh WD, Suah FBM (2021) Chitosan modifications for adsorption of pollutants — a review. J Hazard Mater 408:124889. https://doi.org/10.1016/j.jhazmat.2020.124889
Awad AM, Shaikh SMR, Jalab R et al (2019) Adsorption of organic pollutants by natural and modified clays: a comprehensive review. Sep Purif Technol 228:115719. https://doi.org/10.1016/j.seppur.2019.115719
Dai Y, Zhang N, Xing C et al (2019) The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223:12–27. https://doi.org/10.1016/j.chemosphere.2019.01.161
Ihsanullah I, Jamal A, Ilyas M et al (2020) Bioremediation of dyes: current status and prospects. J Water Process Eng 38:101680. https://doi.org/10.1016/j.jwpe.2020.101680
Omer AM, Dey R, Eltaweil AS et al (2022) Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab J Chem 15:103543. https://doi.org/10.1016/j.arabjc.2021.103543
Gupta G, Khan J, Singh NK (2021) Application and efficacy of low-cost adsorbents for metal removal from contaminated water: a review. Mater Today Proc 43:2958–2964. https://doi.org/10.1016/j.matpr.2021.01.316
Bilal M, Ihsanullah I, Ul Hassan Shah M, Younas M (2021) Enhanced removal of cadmium from water using bio-sorbents synthesized from branches and leaves of Capparis decidua and Ziziphus mauritiana. Environ Technol Innov 24:101922. https://doi.org/10.1016/j.eti.2021.101922
Olabanji IO, Oluyemi EA (2021) Investigating the effectiveness of raw okra (Abelmoschus esculentus L) and raw sugarcane (Saccharum officinarum) wastes as bioadsorbent of heavy metal in aqueous systems. Modern Adv Geogr Environ Earth Sci 6:50–58
Liu Y, Li X, Zhou J, He W (2019) Preparation and characterization of Camellia oleifera nut shell-based bioadsorbent and its application for heavy metals removal. BioResources 14:234–250
Hernández-Cocoletzi H, Salinas RA, Águila-Almanza E et al (2020) Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ Technol Innov 20:101109
Guo S, Jiao P, Dan Z et al (2017) Synthesis of magnetic bioadsorbent for adsorption of Zn (II), Cd (II) and Pb (II) ions from aqueous solution. Chem Eng Res Des 126:217–231
de Andrade Neto JC, Pereira GJ, Morandim-Giannetti AdeA (2020) Chitosan and corn stover derivative bioadsorbent: characterization and application in hexavalent chromium adsorption processes. Cellulose 27:6317–6331
Janyasuthiwong S, Phiri SM, Kijjanapanich P et al (2015) Copper, lead and zinc removal from metal-contaminated wastewater by adsorption onto agricultural wastes. Environ Technol 36:3071–3083. https://doi.org/10.1080/09593330.2015.1053537
Alalwan HA, Kadhom MA, Alminshid AH (2020) Removal of heavy metals from wastewater using agricultural byproducts. J Water Supply Res Technol AQUA 69:99–112. https://doi.org/10.2166/aqua.2020.133
Tsade H, Murthy HA, Muniswamy D (2020) Bio-sorbents from agricultural wastes for eradication of heavy metals: a review. J Mater Environ Sci 11:1719–1735
Nieva AD, Camus REE, Halabasco ER, Doma BT (2020) Prediction of adsorptive capacity of various agricultural wastes in the removal of heavy metals dyes and antibiotic in wastewater using ANN. Int J Environ Sci Dev 11
Ibrahim BM (2021) Heavy metal ions removal from wastewater using various low-cost agricultural wastes as adsorbents: a survey. Zanco J Pure Appl Sci 33:76–91
Minkova V, Razvigorova M, Bjornbom E et al (2001) Effect of water vapour and biomass nature on the yield and quality of the pyrolysis products from biomass. Fuel Process Technol 70:53–61. https://doi.org/10.1016/S0378-3820(00)00153-3
Šćiban M, Klašnja M, Škrbić B (2008) Adsorption of copper ions from water by modified agricultural by-products. Desalination 229:170–180
Kahraman S, Dogan N, Erdemoglu S (2008) Use of various agricultural wastes for the removal of heavy metal ions. Int J Environ Pollut 34:275–284
Bandela NN, Babrekar M, Jogdand O, Kaushik G (2016) Removal of copper from aqueous solution using local agricultural wastes as low cost adsorbent. J Mater Environ Sci 7:1972–1978
Al Bsoul A, Zeatoun L, Abdelhay A, Chiha M (2014) Adsorption of copper ions from water by different types of natural seed materials. Desalin Water Treat 52:5876–5882
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74. https://doi.org/10.1016/j.seppur.2003.10.004
Boukhlifi F, Bencheikh A, Ahlafi H (2011) Characterisation and adsorption propriety of chitin treated at high temperature Caracterisation et proprietes d’adsorption de la chitine traitee a haute temperature. Phys Chem News 58:67–72
Fatima B (2020) Quantitative Analysis by IR: Determination of Chitin/Chitosan DD. Mod Spectrosc Techn Appl. https://doi.org/10.5772/intechopen.89708
Nasernejad B, Zadeh TE, Pour BB et al (2005) Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process Biochem 40:1319–1322. https://doi.org/10.1016/j.procbio.2004.06.010
Marshall WE, Champagne ET (1995) Agricultural byproducts as adsorbents for metal ions in laboratory prepared solutions and in manufacturing wastewater. J Environ Sci Health A Environ Sci Eng Toxicol 30:241–261. https://doi.org/10.1080/10934529509376198
Lee SH, Jung CH, Chung H et al (1998) Removal of heavy metals from aqueous solution by apple residues. Process Biochem 33:205–211. https://doi.org/10.1016/S0032-9592(97)00055-1
Low KS, Lee CK, Leo AC (1995) Removal of metals from electroplating wastes using banana pith. Biores Technol 51:227–231. https://doi.org/10.1016/0960-8524(94)00123-I
Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Sep Purif Technol 43:1–8. https://doi.org/10.1016/j.seppur.2004.09.003
Villaescusa I, Fiol N, Martínez M et al (2004) Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res 38:992–1002. https://doi.org/10.1016/j.watres.2003.10.040
Basci N, Kocadagistan E, Kocadagistan B (2004) Biosorption of copper (II) from aqueous solutions by wheat shell. Desalination 164:135–140. https://doi.org/10.1016/S0011-9164(04)00172-9
Çay S, Uyanik A, Özaşik A (2004) Single and binary component adsorption of copper(II) andcadmium(II) from aqueous solutions using tea-industry waste. Sep Purif Technol 38:273–280. https://doi.org/10.1016/j.seppur.2003.12.003
Sun G, Shi W (1998) Sunflower stalks as adsorbents for the removal of metal ions from wastewater. Ind Eng Chem Res 37:1324–1328. https://doi.org/10.1021/ie970468j
Ho YS (2003) Removal of copper ions from aqueous solution by tree fern. Water Res 37:2323–2330. https://doi.org/10.1016/S0043-1354(03)00002-2
Özcan A, Özcan AS, Tunali S et al (2005) Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper(II) ions onto seeds of Capsicum annuum. J Hazard Mater 124:200–208. https://doi.org/10.1016/j.jhazmat.2005.05.007
Ho YS, Ofomaja AE (2006) Kinetic studies of copper ion adsorption on palm kernel fibre. J Hazard Mater 137:1796–1802. https://doi.org/10.1016/j.jhazmat.2006.05.023
Altun T, Pehlivan E (2007) Removal of copper (II) ions from aqueous solutions by walnut, hazelnutand almond shells. Clean Soil Air Water 35:601–606
Liu H, Wang Y, Li Q et al (2022) Research on the evolution characteristics of oxygen-containing functional groups during the combustion process of the torrefied corn stalk. Biomass Bioenerg 158:106343. https://doi.org/10.1016/j.biombioe.2022.106343
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143
Li G, Huang S, Zhu N et al (2021) Defect-rich heterojunction photocatalyst originated from the removal of chloride ions and its degradation mechanism of norfloxacin. Chem Eng J 421:127852. https://doi.org/10.1016/j.cej.2020.127852
Urruzola I, Robles E, Serrano L, Labidi J (2014) Nanopaper from almond (Prunus dulcis) shell. Cellulose 21:1619–1629. https://doi.org/10.1007/s10570-014-0238-y
Ertaş M, Alma MH (2010) Pyrolysis of laurel (Laurus nobilis L.) extraction residues in a fixed-bed reactor: Characterization of bio-oil and bio-char. J Anal Appl Pyrol 88:22–29
Kurosaki F, Koyanaka H, Hata T, Imamura Y (2007) Macroporous carbon prepared by flash heating of sawdust. Carbon (New York, NY) 45:671–673
Malika A, Jacques N, Jaafar EF et al (2016) Pyrolysis investigation of food wastes by TG-MS-DSC technique. Biomass Conversion Biorefinery 6:161–172
Cruz G, Rodrigues AdaLP, da Silva DF, Gomes WC (2021) Physical–chemical characterization and thermal behavior of cassava harvest waste for application in thermochemical processes. J Therm Anal Calorim 143:3611–3622. https://doi.org/10.1007/s10973-020-09330-6
Ahmadou A, Napoli A, Durand N, Montet D (2019) High physical properties of cashew nut shell biochars in the adsorbtion of mycotoxins. Inter J Food Res 6:18
Ahmadou A, Brun N, Napoli A et al (2019) Effect of pyrolysis temperature on ochratoxin A adsorption mechanisms and kinetics by cashew nut shell biochars. SRDP J Food Sci Tech 4:877
Annab H, Fiol N, Villaescusa I, Essamri A (2019) A proposal for the sustainable treatment and valorisation of olive mill wastes. J Environ Chem Eng 7:102803. https://doi.org/10.1016/j.jece.2018.11.047
Bautista-Toledo I, Ferro-García MA, Rivera-Utrilla J et al (2005) Bisphenol A removal from water by activated carbon. Effects of carbon characteristics and solution chemistry. Environ Sci Technol 39:6246–6250. https://doi.org/10.1021/es0481169
Yu B, Zhang Y, Shukla A et al (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption — removal of copper. J Hazard Mater 80:33–42. https://doi.org/10.1016/S0304-3894(00)00278-8
Vishnu D, Dhandapani B, Ramakrishnan SR et al (2021) Fabrication of surface-engineered superparamagnetic nanocomposites (Co/Fe/Mn) with biochar from groundnut waste residues for the elimination of copper and lead metal ions. J Nanostruct Chem 11:215–228. https://doi.org/10.1007/s40097-020-00360-y
Vishnu D, Dhandapani B (2021) Synthesis of novel adsorbent by incorporation of plant extracts in amino-functionalized silica-coated magnetic nanomaterial for the removal of Zn2+ and Cu2+ from aqueous solution. J Environ Health Sci Eng 19:1413–1424. https://doi.org/10.1007/s40201-021-00696-9
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Handlingar 24:1
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Ganesan P, Lakshmi J, Sozhan G, Vasudevan S (2013) Removal of manganese from water by electrocoagulation: adsorption, kinetics and thermodynamic studies. Can J Chem Eng 91:448–458. https://doi.org/10.1002/cjce.21709
Badawi MA, Negm NA, Abou Kana MTH et al (2017) Adsorption of aluminum and lead from wastewater by chitosan-tannic acid modified biopolymers: isotherms, kinetics, thermodynamics and process mechanism. Int J Biol Macromol 99:465–476. https://doi.org/10.1016/j.ijbiomac.2017.03.003
Rai MK, Giri BS, Nath Y et al (2018) Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from almond shell: kinetics, equilibrium and thermodynamics study. J Water Supply Res Technol AQUA 67:724–737. https://doi.org/10.2166/aqua.2018.047
Homem V, Alves A, Santos L (2010) Amoxicillin removal from aqueous matrices by sorption with almond shell ashes. Int J Environ Anal Chem 90:1063–1084. https://doi.org/10.1080/03067310903410964
Thitame PV, Shukla SR (2017) Removal of lead (II) from synthetic solution and industry wastewater using almond shell activated carbon. Environ Prog Sustainable Energy 36:1628–1633. https://doi.org/10.1002/ep.12616
Fat’hi MR, Asfaram A, Hadipour A, Roosta M (2014) Kinetics and thermodynamic studies for removal of acid blue 129 from aqueous solution by almond shell. J Environ Health Sci Eng 12:62. https://doi.org/10.1186/2052-336X-12-62
Vishnu D, Dhandapani B, Vaishnavi G, Preethi V (2022) Synthesis of tri-metallic surface engineered nanobiochar from cynodon dactylon residues in a single step — batch and column studies for the removal of copper and lead ions. Chemosphere 286:131572. https://doi.org/10.1016/j.chemosphere.2021.131572
Vishnu D, Dhandapani B, Santhiya K (2021) The symbiotic effect of integrated Muraya koenigii extract and surface-modified magnetic microspheres — a green biosorbent for the removal of Cu(II) and Cr(VI) ions from aqueous solutions. Chem Eng Commun 208:851–862. https://doi.org/10.1080/00986445.2019.1691538
Marshall WE, Champagne ET (1995) Agricultural byproducts as adsorbents for metal ions in laboratory prepared solutions and in manufacturing wastewater. J Environ Sci Health A 30:241–261
Aksu Z, İşoğlu İA (2005) Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem 40:3031–3044
Altun T, Pehlivan E (2007) Removal of copper (II) ions from aqueous solutions by walnut-, hazelnut-and almond-shells. Clean Soil Air Water 35:601–606
Ge Y, Li Z, Xiao D et al (2014) Sulfonated multi-walled carbon nanotubes for the removal of copper (II) from aqueous solutions. J Ind Eng Chem 20:1765–1771. https://doi.org/10.1016/j.jiec.2013.08.030
Yu C, Shao Z, Liu L, Hou H (2018) Efficient and selective removal of copper(II) from aqueous solution by a highly stable hydrogen-bonded metal–organic dramework. Cryst Growth Des 18:3082–3088. https://doi.org/10.1021/acs.cgd.8b00224
Yusuf M, Khan MA, Abdullah EC et al (2016) Dodecyl sulfate chain anchored mesoporous graphene: Synthesis and application to sequester heavy metal ions from aqueous phase. Chem Eng J 304:431–439. https://doi.org/10.1016/j.cej.2016.06.109
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide– chitosan composite hydrogels as broad- spectrum adsorbents for water purification. J Mater Chem A 1:1992–2001. https://doi.org/10.1039/C2TA00406B
Katiyar R, Patel AK, Nguyen T-B et al (2021) Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Biores Technol 328:124829. https://doi.org/10.1016/j.biortech.2021.124829
Funding
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: 20- SCI-1–01-0006.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kali, A., Amar, A., Loulidi, I. et al. Characterization and adsorption capacity of four low-cost adsorbents based on coconut, almond, walnut, and peanut shells for copper removal. Biomass Conv. Bioref. 14, 3655–3666 (2024). https://doi.org/10.1007/s13399-022-02564-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02564-4