Abstract
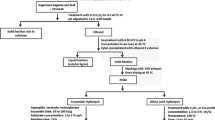
In the present study, xylooligosaccharides (XOS) production was carried out using sorghum (Sorghum bicolor (L.)) xylan and xylanase from isolated Aspergillus fumigates RSP-8 followed by evaluation of their prebiotic potential using Lactobacillus sp. T-10. Isolated xylan revealed structural similarity to Birchwood xylan and is composed of xylose (89%) arabinose (6%) and glucose (5%). Xylanase enzyme produced by Aspergillus fumigatus RSP-8 revealed 43 kDa molecular weight with little β-glucosidase and depicted optimum activity of 1248 U/mL at pH 5.0 and temp 50 °C. Time-dependent XOS [(X2 (xylobiose) to X7 (xyloheptose)] production was noticed and differed from source to source. XOS production kinetics indicated that maximum XOS (8.4 g reducing sugar/L) was obtained in 12 h at 50 °C with 50 U/mL xylanase with 1.5% (w/v) sorghum xylan as substrate. The produced XOS promoted Lactobacillus T-10 growth indicating its prebiotic potential. This study can provide useful insights into the processes required for further upscaling of agricultural biomass to nutraceutical product production and the industrial application potential of sorghum xylan.









Similar content being viewed by others

References
Farzad S, Mandegari MA, GUo M, Haigh KF, Shah N, Gorgens JF, (2017) Multi-product biorefineries from lignocelluloses: a pathway to revitalization of the sugar industry. Biotechnol Biofuels 10(1):1–24. https://doi.org/10.1186/s13068-017-0761-9
Hoang NV, Furtado A, Donnan L, Keeffe EC, Botha FC, Henry RJ (2017) High-throughput profiling of the fiber and sugar composition of sugarcane biomass. Bioenerg Res 10(2):400–416. https://doi.org/10.1007/s12155-016-9801-8
Peng F, Ren JL, Xu F, Bian J, Peng P, Sun RC (2012) Fractional study of alkali-soluble hemicelluloses obtained by graded ethanol precipitation from sugar cane bagasse. J agric food chem 58(3):1768–1776. https://doi.org/10.1021/jf9033255
Nicolas MC, Giselle G, Carolina MM, Julia K, Maria CA, Maria EV (2021) Biomass waste as sustainable raw material for energy and fuels. Sustainability 13(2):1–21. https://doi.org/10.3390/su13020794
Davison BH, Drescher SR, Tuskan GA, Davis MF, Nghiem NP (2006) Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl Biochem Biotechnol 130(1):427–435. https://doi.org/10.1385/abab:130:1:427
Sathish T, Prakasham RS (2013) Intensification of fructosyl transferases and fructo-oligosaccharides production in solid state fermentation by Aspergillus awamori GHRTS. Indian J microbiol 53(3):337–342. https://doi.org/10.1007/2Fs12088-013-0380-5
Chiranjeevi T, Uma A, Radhika K, Babyrani G, Prakasham RS, Srinivasa rao P, Umaknth AV, (2014) Enzymatic hydrolysis of market vegetable waste and subsequent ethanol fermentation-kinetic evaluation. J Biochem Tech 5(4):775–781
Raj T, Kapoor M, Gaur R, Christopher J, Lamba B, Tuli DK, Kumar R (2015) Physical and chemical characterization of various Indian agricultural residues for biofuels production. Energy Fuels 29(5):3111–3118. https://doi.org/10.1021/ef5027373
Bhatia R, Winters A, Bryant DN, Bosch M, Clifton-Brown J, Leak D, Gallagher J (2020) Pilot-scale production of xylo-oligosaccharides and fermentable sugars from Miscanthus using steam explosion pretreatment. Bioresour Technol 296:1–9. https://doi.org/10.1016/j.biortech.2019.122285
Pauly M, Keegstra K (2008) Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J 54(4):559–568. https://doi.org/10.1111/j.1365-313X.2008.03463.x
Ruiz HA, Miguel AC, Helder DS, Rosa MRJ, Antonio AV, Jose AT (2013) Biorefinery valorization of autohydrolysis wheat straw hemicelluloses to be applied in a polymer–blend film. Carbohydr Polym 92(2):2154–2162. https://doi.org/10.1016/j.carbpol.2012.11.054
Cesar DPM, Rosa MRJ, Rafel GA, Araceli LT, Debora N, Beatriz G, Hector AR (2021) Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: a review. Ind Crop Prod 162:113274. https://doi.org/10.1016/j.indcrop.2021.113274
Aguedo M, Ruiz HA, Richel A (2015) Non alkaline solubilization of arabinoxylans from destarched wheat branusing hydrothermal microwave processing and comparison with hydrolysis by an endoxylanase. Chem Eng Process 96:72–82. https://doi.org/10.1016/j.cep.2015.07.020
Gullon B, Davil I Garcia TM, Yanez R, Labidi J, Gullon P (2017) Production and emerging applications of bioactive oligosaccharides from biomass hemicelluloses by hydrothermal processing. In: Ruiz H, Hedegaard TM, Trajano H. (ed) Hydrothermal processing in biorefineries. Springer, Cham. https://doi.org/10.1007/978-3-319-56457-9_10
Bhardwaj N, Kumar B, Verma P (2019) A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess 6(1):1–36. https://doi.org/10.1186/s40643-019-0276-2
Ebringerova A, Heinze T (2000) Xylan and xylan derivatives-biopolymers with valuable properties, 1: naturally occurring xylans structures, isolation procedures and properties. Macromole Rapid commun 21(9):542–556. https://doi.org/10.1002/1521-3927(20000601)21:9/3C542::AID-MARC542/3E3.0.CO;2-7
Dodd D, cann IK, (2009) Enzymatic deconstruction of xylan for biofuel production. Gcb Bioenergy 1(1):2–17. https://doi.org/10.1111/j.1757-1707.2009.01004.x
Walia A, Guleria S, Mehta P, Chauhan A, Prakash J (2017) Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3Biotech 7(1):1–12. https://doi.org/10.1007/s13205-016-0584-6
Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) xylanases from fungi: Properties and industrial applications. Appl Microbiol Biotechnol 67(5):577–591. https://doi.org/10.1007/s00253-005-1904-7
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker DLAP (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure, NREL, India
Sharma K, Morla S, Khaire KC, Thakur A, Moholkar VS, Kumar S, Goyal A (2020) Extraction, characterization of xylan from Azadirachta indica (neem) saw dust and production of anti-proliferative xylooligosaccharides. Int J Biol Macromol 163:1897–1907. https://doi.org/10.1016/j.ijbiomac.2020.09.086
Sun SN, Cao XF, Sun RC, Jones GL, Baird M (2014) Structural and thermal property of alkaline hemicelluloses from steam exploded Phyllostachys pubescens. Carbohydr poly 101:1191–1197. https://doi.org/10.1016/j.carbpol.2013.09.109
Bakri Y, Akeed Y, Jawhar M, Arabi MIE (2020) Evaluation of xylanases production from filamentous fungi with different lifestyles. Acta Aliment 49(2):197–203. https://doi.org/10.1556/066.2020.49.2.9
Capek P, Matulova M (2013) An arabino (glucuroa) xylan isolated from immunomodulatory active hemicelluloses fraction of Salvia officinalis. L Int J of Biol Macromol 59:396–401
Anuj C, Silva SSD (2013) Sustainable degradation of lignocellulosic biomass: techniques, applications. In: Motta FL, Andrade CCP,Santana MHA (ed) A review of xylanase production by the fermentation of xylan: classification, characterization and applications. Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization 10:251–274. https://doi.org/10.5772/53544
Qi L, Yunpeng J, Xinyi T, Linguo Z, Jianjun P (2021) Co-production of xylooligosaccharides and xylose from poplar saw dust by Recombinant Endol-1,4-βxylanase and β-Xylosidase mixture hydrolysis. Front Bioeng Biotechnol 8:1–13. https://doi.org/10.3389/2Ffbioe.2020.637397
Cano ME, Garcia MA, Comendador MP, Wojtusik M, Santos VE, Kovensky J, Ladero M (2020) Production of oligosaccharides from agrofood wastes. Fermentation 6:31. https://doi.org/10.3390/fermentation6010031
Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2008) Genes and molecules of Lactobacilli supporting probiotic action. Macrobiol Molecular biol Rev 72(4):728–764. https://doi.org/10.1128/MMBR.00017-08
Prakasham RS, Nagaiah D, Vinutha KS, Uma A, Chiranjeevi T, Umakanth AV, Rao PS, Yan N (2014) Sorghum biomass: a novel renewable carbon sources for industrial bioproducts. Biofuels 5(2):159–174. https://doi.org/10.4155/bfs.13.74
Kacurakova M, Hirisch EA, Hromadkova J, Z, (1994) Infrared study of arabinoxylans. J Sci Food Agric 66(3):423–427. https://doi.org/10.1002/jsfa.2740660323
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23(3):257–270. https://doi.org/10.1016/0168-1656(92)90074-J
Schmoll M (2018) Regulation of plant cell wall degradation by light in Trichoderma. Fungal Biol Biotechnol 5(1):1–20. https://doi.org/10.1186/s40694-018-0052-7
Deshpande AP, Bhaskarrao M, Lakshmana rao C (2000) Extraction of bamboo fibers and their use as reinforcement in polymeric composites. J Appl Poly Sci 76(1):83–92. https://doi.org/10.1002/(SICI)1097-4628(20000404)76:1/3C83::AID-APP11/3E3.0.CO;2-L
Huisman MMH, Schols HA, Voragen AGJ (2000) Glucuronoarabinoxylans from maize kernel cell walls are more complex than those from sorghum kernel cell walls. Carbohydr poly 43(3):269–279. https://doi.org/10.1016/S0144-8617(00)00154-5
Berth G, Dautzenberg H, Peter MG (1998) Physico-chemical characterization of chitosans varying in degree of acetylation. Carbohydr Polym 36(2–3):205–216. https://doi.org/10.1016/S0144-8617(98)00029-0
Robledo MT, Calvo C, Aranda E (2020) Enzymatic potential of bacteria and fungal isolates from the sewage sludge composting process. Appl Sci 10(21):7763. https://doi.org/10.3390/app10217763
Ak S, Jayapal N, Chikkerur J, Roy S, Kolte A, Senani S, Sridhar M (2015) Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioact Carbohydr Diet Fibre 5:62–71. https://doi.org/10.1016/j.bcdf.2014.12.003
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. Fems Microbiol Rev 23(4):411–456. https://doi.org/10.1111/j.1574-6976.1999.tb00407.x
Ravichandra K, Yaswanth VVN, Nikhila B, Ahmad J, Rao PS, Uma A, Ravindrababu V, Prakasham RS (2016) Xylanase production by isolated fungal strain Aspergillus fumigatus RSP-8 (MTCC 12039): impact of agro-industrial material as substrate. Sugar tech 18(1):29–38. https://doi.org/10.1007/s12355-014-0357-7
Biely P, Kratky Z, Vrsanka M, Urmanicova D (1981) Induction and inducers of endo-1,4-β-xylanase in the yeast Cryptococcus Albidus. In: Hollaender A, Rabson R,Rogers P, Pietro AS, Valentine R, wolfe R (ed) Trends in the biology of fermentation for fuels and chemicals. Basic life sciences. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-3980-9_36
Moretti M, Bocchini MDA, Silva RD, Rodrigues A, Sette LD, Gomes E (2012) Selection of thermophilic and thermos tolerant fungi for the production of cellulases and xylanases under solid state fermentation. Braz J Microbiol 43(3):1062–1071. https://doi.org/10.1590/S1517-83822012000300032
Adhikari L, Missaoui AM (2019) Quantitative trait loci mapping of leaf rust resistance in tetraploid alfalfa. Physiol Mol Plant Path 106:238–245. https://doi.org/10.1016/j.pmpp.2019.02.006
Aachary AA, Prapulla SG (2009) Value addition to corncob: production and characterization of xylooligosaccharides from alkali treated lignin- saccharide complex using Aspergillus oryzae MTCC 5154. Bioresour Technol 100(2):991–995. https://doi.org/10.1016/j.biortech.2008.06.050
Agro N, Pakula T, Penttila M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29(4):719–739. https://doi.org/10.1016/j.femsre.2004.11.006
Akpinar O, Bostanci EK, S, (2009) Enzymatic production of xylooligosaccharides from selected agricultural wastes. Food Bioprod Process 87(2):145–151. https://doi.org/10.1016/j.fbp.2008.09.002
Lasrado LD, Gudipati M (2015) Antioxidant property of symbiotic combination of Lactobacillus sp and wheat bran xylo-oligosaccharides. J Food Sci Technol 52(7):4551–4557. https://doi.org/10.1007/s13197-014-1481-9
Iliev I, Vasileva T, Bivolarski V, Momchilova A, Ivanova I (2020) Metabolic profiling of xylo-oligosaccharides by Lactobacilli. Polymers 12(10):2387. https://doi.org/10.3390/polym12102387
Ananieva M, Mandzhieva T,Stojanovski S , Ilia I, Ivanova I (2012) Utilization of xylooligosaccharides from different Lactobacillus strains J Biosci Biotech 147–150.http://eprints.uklo.edu.mk/id/eprint/6242
Li Z, Summanen PH, Komoriya T, Finegold SM (2015) In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp. Int J Food Sci Nutr 66(8):919–922. https://doi.org/10.3109/09637486.2015.1064869
Acknowledgements
The authors are grateful to the Director, CSIR-IICT Hyderabad, for providing facilities to carry out this work and Council of Scientific and Industrial Research, Government of India, for the award of Research Fellowship to K. Ravi Chandra. The manuscript communication number through CSIR-IICT is IICT/Pubs./2020/347.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ravichandra, K., Balaji, R., Devarapalli, K. et al. Enzymatic production of prebiotic xylooligosaccharides from sorghum (Sorghum bicolor (L.) xylan: value addition to sorghum bagasse. Biomass Conv. Bioref. 13, 11131–11139 (2023). https://doi.org/10.1007/s13399-021-02216-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02216-z