Abstract
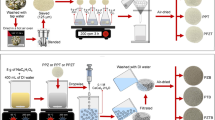
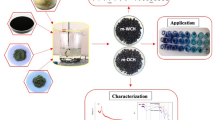
Dyes are noxious organic compounds released from different sources producing severe water pollution, thus imparting color to water consequently affecting ecosystem, terrestrial, as well as aquatic life and reducing transmission of sunlight through water. Several techniques have been used for wastewater treatment but adsorption has been proposed as a most effective and significant method for toxic dye removal from industrial wastewater. Low-cost and easily available biomass can be used for the wastewater treatment. The purpose of this study is to synthesize and use of cost-effective, eco-friendly, easily accessible, and magnetically modified nano-biosorbents of pine needles (MCPN) and banana peels (MCBP) for methylene blue dye adsorption from aqueous and industrial effluent. The characterization of prepared nano-composite in terms of structure, morphology, composition and thermal stability were well supported by field emission scanning electron microscopy, X-ray diffraction pattern, Fourier transform infrared spectroscopy, and thermogravimetric analysis. The adsorption experimentations were investigated in batch sorption mode to examine the influence of pH, adsorbent dosage, contact time, concentration of dye, and temperature. The kinetics of the adsorption study followed the pseudo–second-order model while the adsorption isotherm data fitted well to the Temkin model for MCBP and the Freundlich model for MCPN. The thermodynamic parameters were measured and proposed that the adsorption processes were spontaneous and endothermic, having increased randomness at the solid–liquid interface during the dye sorption. The adsorption capacity of MCPN equipped under optimized conditions for methylene blue was 1193 and 296.4 mg/g for MCBP. Moreover, the successful application in industrial effluent of MCPN showed more adsorption as compared to MCBP. In conclusion, research elucidated that magnetically modified biosorbents could be used to remove methylene blue dye from wastewater to improve water quality and overcome disposal problem and waste management in an eco-friendly way.










Similar content being viewed by others
References
Sharma D, Goel G, Sud A, Chauhan RS (2015) A novel laccase from newly isolated Cotylidia pannosa and its application in decolorization of synthetic dyes. Biocatal Agri Biotechnol 4:661–666
Cestari AR, Vieira EF, Vieira GS, Almeida LE (2007) Aggregation and adsorption of reactive dyes in the presence of an anionic surfactant on mesoporous aminopropyl silica. J Col Int Sci 309:402–411
Al-Degs Y, Khraisheh M, Allen S, Ahmad M (2001) Sorption behavior of cationic and anionic dyes from aqueous solution on different types of activated carbons. Sep Sci Technol 36:91–102
Zeng G, Cheng M, Huang D, Lai C, Xu P, Wei Z, Li N, Zhang C, He X, He Y (2015) Study of the degradation of methylene blue by semi-solid-state fermentation of agricultural residues with Phanerochaete chrysosporium and reutilization of fermented residues. J Clean Prod 38:424–430
Chowdhury S, Bhattacharyya KG (2019) Use of Cu (II)-incorporated zeolite Y for decolourization of dyes in water: a case study with aqueous methylene blue and Congo red. J Appl Sci 1(87):102
Saratale RG, Saratale GD, Chang J, Govindwar S (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Vieira M, de Almeida Neto A, Da Silva M, Carneiro C, Melo Filho A (2014) Adsorption of lead and copper ions from aqueous effluents on rice husk ash in a dynamic system. J Chem Eng 31:519–529
Morioka T, Tokai A, Yamamoto Y, Matsui T (2010) Selection of product categories for a national eco-labelling scheme in developing countries: a case study of Vietnamese manufacturing sub-sectors. J Clean Prod 18:1446–1457
Tang, R., Dai, C., Li, C., Liu, W., Gao, S. & Wang, C. (2017). Removal of methylene blue from aqueous solution using agricultural residue walnut shell: equilibrium, kinetic, and thermodynamic studies. J Chem 2017
El-Naggar I, Ahmed SA, Shehata N, Sheneshen E, Fathy M, Shehata A (2019) A novel approach for the removal of lead (II) ion from wastewater using kaolinite/smectite natural composite adsorbent. Appl Water Sci 9:7–83
Ben-Ali S, Jaouali I, Souissi-Najar S, Ouederni A (2017) Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J Clean Prod 142:3809–3821
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Ngo HH, Guo W, Zhang J, Liang S, Ton-That C, Zhang X (2015) Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour Technol 182:353–363
Ajab H, Khatoon M, Yaqub A, Gulfaraz M, Nawazish S, Khan F. A., Dennis J. O., Junaid M. (2021). Synthesis of Gallus gallus domesticus eggshells and magnetite bio-composite for Cr(VI) removal: adsorption kinetics, equilibrium isotherms and thermodynamics. Desalin Water Treat. (in press)
Kim N, Park M, Park D (2015) A new efficient forest biowaste as biosorbent for removal of cationic heavy metals. Bioresour Technol 175:629–632
Carpenter CD, O’Neill T, Picot N, Johnson JA, Robichaud GA, Webster D, Gray CA (2012) Anti-mycobacterial natural products from the Canadian medicinal plant Juniperus communis. J Clean Prod Technol 143:695–700
Ahmad M, Lee SS, Rajapaksha AU, Vithanage M, Zhang M, Cho JS, Lee S-E, Ok YS (2013) Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour Technol 3:615–622
Chen B, Yuan M, Liu H (2011) Removal of polycyclic aromatic hydrocarbons from aqueous solution using plant residue materials as a biosorbent. J Hazard Mater 188:436–442
Khoozani AA, Birch J, Bekhit AE-DA (2019) Production, application and health effects of banana pulp and peel flour in the food industry. J Food Sci Technol 56:548–559
Wu R, Wang Y, Xue X, Hu T, Gao J, An F (2019) Selective adsorption and removal ability of pine needle-based activated carbon towards Al (III) from La (III). J Dis Sci Technol 40:186–191
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Degefu DM, Dawit M (2013) Chromium removal from Modjo Tannery wastewater using Moringa stenopetala seed powder as an adsorbent. Water Air Soil Pollut 224(12):1719
Chen H, Wageh S, Al-Ghamdi AA, Wang H, Yu J, Jiang C (2019) Hierarchical C/NiO-ZnO nanocomposite fibers with enhanced adsorption capacity for Congo red. J Coll Inter Sci 537:736–745
Chojnacka K (2010) Biosorption and bioaccumulation–the prospects for practical applications. Inter Environ Chem 36:299–307
Kong W, Zhao F, Guan H, Zhao Y, Zhang H, Zhang B (2016) Highly adsorptive mesoporous carbon from biomass using molten-salt route. J Mater Sci 51(14):6793–6800
Meng Su, Yan W, Ma X, Sun D, Jin Y, He K (2019) Hierarchical structured Mn2O3 nanomaterials with excellent electrochemical properties for lithium ion batteries.". RSC Adv 9(3):1284–1289
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Coll Surf Sci 368:58–63
Chen X, Zhang N, Sun K (2012) Facile fabrication of CuO 1D pine-needle-like arrays for super-rate lithium storage. J Mater Chem 22:15080–15084
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated synthesis of gold nanoparticles. Coll Surf Inter 80:45–50
Al-Mohammedawi HH, Znad H, Eroglu E (2019) Improvement of photofermentative biohydrogen production using pre-treated brewery wastewater with banana peels waste. J Hyd Eng 44:2560–2568
Aksu Z, Tatlı Aİ, Tunç Ö (2008) A comparative adsorption/biosorption study of Acid Blue 161: effect of temperature on equilibrium and kinetic parameters.". Chem Eng J 142(1):23–39
Asma N, Saad B, Bhat AH, Khan AS, Danish M, Isa MH, Naeem A (2019) Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: characterization and application for methylene blue removal. J Clean Prod 211:1190–1200
Wong S, Tumari HH, Ngadi N, Mohamed NB, Hassan O, Mat R, Amin NAS (2019) Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine. J Clean Prod 206:394–406
Alencar Wagner S, Acayanka Elie, Lima Eder C, Royer Betina, de Souza Felipe E, Lameira Jerônimo, Alves Cláudio N (2012) Application of Mangifera indica (mango) seeds as a biosorbent for removal of Victazol Orange 3R dye from aqueous solution and study of the biosorption mechanism. Chem. Eng. J. 209:577–588
Feisther VA, Scherer Filho J, Hackbarth FV, Mayer DA, de Souza AAU, de Souza SMGU (2019) Raw leaves and leaf residues from the extraction of essential oils as biosorbents for metal removal. J. Environ. Chem. Eng. 7:103047
Cherdchoo W, Nithettham S, Charoenpanich J (2019) Removal of Cr (VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 221:758–767
Langmuir I (1917) The constitution and fundamental properties of solids and liquids. II. Liquids. J Am Chem Soc 39(9):1848–1906
Rathod M., Mody K., Basha S (2014) Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alvarezii. J Clean Prod 84 484e493
Li Y, Taggart MA, McKenzie C, Zhang Z, Lu Y, Pap S, Gibb S (2019) Utilizing low-cost natural waste for the removal of pharmaceuticals from water: mechanisms, isotherms and kinetics at low concentrations. J Clean Prod 227:88–97
Lee LY, Gan S, Tan MSY, Lim SS, Lee XJ, Lam YF (2016) Effective removal of Acid Blue 113 dye using overripe Cucumis sativus peel as an eco-friendly biosorbent from agricultural residue. J Clean Prod 113:194–203
Ronix A, Pezoti O, Souza LS, Souza IP, Bedin KC, Souza PS, Silva TL, Melo SA, Cazetta AL, Almeida VC (2017) Hydrothermal carbonization of coffee husk: optimization of experimental parameters and adsorption of methylene blue dye. J Environ Chem Eng 5(5):4841–4849
Rahman MA, Amin SR, Alam AS (2012) Removal of methylene blue from waste water using activated carbon prepared from rice husk. Dhaka University Journal of Science 60(2):185–189
Mohammed M, Shitu A, Ibrahim A (2014) Removal of methylene blue using low cost adsorbent: a review. Res J Chem Sci 2231:606X
Linda BLL, Namal P, Tennakoon DTB, Hei IC, Muhammad KD, Montri S (2017) Breadnut peel as a highly effective low-cost biosorbent for methylene blue: equilibrium, thermodynamic and kinetic studies. Arab J Chem 10:S3216–S3228
Chieng HI, Zehra T, Lim LB, Priyantha N, Tennakoon DTB (2014) Sorption characteristics of peat of Brunei Darussalam IV: equilibrium, thermodynamics and kinetics of adsorption of methylene blue and malachite green dyes from aqueous solution. Environ Earth Sci 72(7):2263–2277
Lim LB, Priyantha N, Hei Ing C, Khairud Dahri M, Tennakoon DTB, Zehra T, Suklueng M (2015) Artocarpus odoratissimus skin as a potential low-cost biosorbent for the removal of methylene blue and methyl violet 2B. Desalin Water Treat 53(4):964–975
Carvalho LB, Chagas PMB, Pinto LMA (2018) Caesalpinia ferrea fruits as a biosorbent for the removal of methylene blue dye from an aqueous medium. Water Air Soil Pollut 229(9):297
Hameed B (2009) Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J Hazard Mater 162(2–3):939–944
Banat F, Al-Asheh S, Al-Makhadmeh L (2003) Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem 39(2):193–202
Chen Y, Zhai SR, Liu N, Song Y, An QD, Song XW (2013) Dye removal of activated carbons prepared from NaOH-pretreated rice husks by low-temperature solution-processed carbonization and H3PO4 activation. Biores Technol 144:401–409
Chen Y, Zhai SR, Liu N, Song Y, An QD, Song XW (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interface Sci 284(1):78–82
Senthil KP, Fernando PSA, Ahmed RT, Srinath R, Priyadharshini M, Vignesh AM, Thanjiappan A et al (2014) Effect of temperature on the adsorption of methylene blue dye onto sulfuric acid–treated orange peel. Chem Eng Commun 201(11):1526–1547
Khan TA, Ali I, Singh VV, Sharma S (2009) Utilization of fly ash as low-cost adsorbent for the removal of methylene blue, malachite green and rhodamine B dyes from textile wastewater. J Environ Protection Sci 3(1):11–22
Fan S, Tang J, Wang Y, Li H, Zhang H, Tang J, Wang Z, Li X (2016) Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: kinetics, isotherm, thermodynamic and mechanism. J Mole Liq 220:432–441
Indira K (2013) Removal of methylene blue dye from aqueous solutions by neem leaf and orange peel powder. Inter J Chem Tech Res 5(2):572–577
Ahmad A, Rafatullah M, Sulaiman O, Ibrahim MH, Hashim R (2009) Scavenging behaviour of meranti sawdust in the removal of methylene blue from aqueous solution. J Hazard Mater 170(1):357–365
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia for funding this work through general research groups program under grant number GRP-6-42.
Author information
Authors and Affiliations
Contributions
Asim Yaqu, Huma Ajab, and Aaqiba Almas work on literature survey; Samia Misbah Syed, Asima Azam, and M Ijaz Khan computed the results; Muhammad Awais, Irshad Muhammad, and Ahmed M Galal wrote the final manuscript; Mohammad Y Alshahrani reviewed the final version.
Corresponding author
Ethics declarations
Ethics approval
The experimental study was approved by the National Veterinary Lab Islamabad ethical committee adhering to the institution’s guidelines and in compliance with ARRIVE guidelines.
Consent to participate
Not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yaqub, A., Ajab, H., Almas, A. et al. Utilization of nano-biosorbents based on pine needles and banana peel for methylene blue removal: equilibrium, kinetics, thermodynamic study, and application. Biomass Conv. Bioref. 12, 1787–1802 (2022). https://doi.org/10.1007/s13399-021-02191-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02191-5