Abstract
The production of biofuels and biochemicals requires a pretreatment to cleave the composite-like structure of lignocellulosic biomass and thus facilitate further conversion. In the case of liquid-based pretreatment, it is important to know which pretreatment liquids allow for an effective conversion of biomass. For the development of effective pretreatment strategies, simple criteria for a fast evaluation of pretreatment results are advantageous. In this study, we use the example of acetosolv pretreatment of beech wood to explore the influence of composition of the employed acetosolv liquids. To this end, we investigate pretreatment phenomena on different scales including macroscopic disintegration, overall mass balances and compositional changes of beech wood. We relate the investigated phenomena with the type and amount of catalyst acid as well as water content of the employed acetosolv liquids. The results show that disintegration increases with both a higher concentration and acidity of the catalyst acid, while excessive disintegration can be balanced by an increased water content up to equimolar ratios of water and acetic acid. Furthermore, an increasing disintegration correlates with an increasing non-recovered fraction up to a maximum of 40 wt%. The non-recovered fraction in turn linearly depends on the amount of removed hemicellulose and lignin. Overall, a low lignin content together with complete disintegration after pretreatment in acetosolv liquids with a high water content allows for increased sugar yields in subsequent enzymatic hydrolysis. Thus, disintegration and non-recovered fraction serve as a simple indicator for a first assessment of pretreatment effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One option for the production of fuels and chemicals in a biorefinery is the conversion of sugars from lignocellulosic biomass [1,2,3,4]. To make these sugars available, a pretreatment is required to cleave the composite-like structure of cellulose, hemicellulose and lignin in combination with acid or enzymatic hydrolysis of the polysaccharides cellulose and hemicellulose [5, 6]. A high sugar yield after enzymatic hydrolysis resulting from an effective pretreatment is important to render a biorefinery economically feasible [7, 8].
Effective biomass pretreatment is associated with a number of changes in morphology and composition of the biomass. In particular, to enhance accessibility of the pretreated biomass for enzymes, an increase in surface area is beneficial. In many cases, this relates to a macroscopic disintegration of biomass after pretreatment [9,10,11]. With regard to biomass composition, a higher fraction of removed lignin mostly correlates with higher sugar yields after enzymatic hydrolysis [12, 13], because lignin not only physically prevents access to cellulose but also inhibits enzymes [14]. Furthermore, decrystallization of cellulose is beneficial for good hydrolyzability [15], although the effect of decrystallization can be balanced by longer hydrolysis times [13]. In many cases, a reduced number of acetyl groups (which are attached to hemicellulose in untreated biomass) after pretreatment positively influences sugar yields [12, 16,17,18]. Nonetheless, the influence of acetyl content on enzymatic digestibility is rated small in comparison to the removal of lignin and decrystallization of cellulose [13].
The above-mentioned phenomena of effective pretreatment (i.e., increased accessible surface area, removal of lignin, decrystallization of cellulose, reduction of acetyl content) are to different extents characteristic for a variety of pretreatment concepts relying on biological, physical or chemical principles [6, 19, 20]. Chemical pretreatment concepts apply electrolytic pretreatment liquids, such as dilute aqueous systems [16], concentrated ionic liquids and deep eutectic solvents [21, 22] or organic solvent-based systems [23, 24]. The organic solvent-based organosolv process enables usage and recovery of both the lignin and the hemicellulose fraction besides the cellulose pulp as a potential pretreatment concept for biorefineries [24, 25]. So far, several variants have been tested including organosolv pretreatment relying on acetic acid as main solvent with the addition of a further catalyst acid, the so-called acetosolv process [26,27,28,29].
The advantage of acetic acid-based pretreatment liquids is an increased solubility for lignin in comparison to alcohols, allowing for pretreatment at mild conditions (i.e., temperatures below 100 ∘C and atmospheric pressure) [28,29,30]. Nevertheless, process conditions such as temperature, duration of the pretreatment and liquid-to-wood ratio appear less important for pulp yield and delignification than the composition of the pretreatment liquid [31,32,33]. In this context, a high concentration of acetic acid is beneficial for an effective removal of lignin [31, 33, 34], while the presence of a catalyst facilitates the removal of lignin in the first place [32, 33]. Nevertheless, the influence of water content on the removal of lignin remains unclear [35]. With regard to biorefinery applications, the influence of varying catalyst types and concentrations and their relation to pretreatment phenomena has not yet been systematically analyzed.
We examine correlations between pretreatment phenomena observed on different length scales with regard to the properties of pretreatment liquids aiming at the identification of simple criteria for the evaluation of pretreatment effectiveness. To this end, beech wood pretreated with a number of acetosolv liquids (i.e., acetic acid with different catalyst acids and varying water contents) serves as an example pretreatment concept. Regarding the composition of pretreatment liquids, we focus on the variation of type and concentration of catalyst acid, while selected additional experiments serve to get a first impression of the effect of a changing water content. The macroscopic separation of wood fibers after acetosolv pretreatment is classified into five newly defined degrees of disintegration as a potential criterion to assess the outcome of pretreatment experiments and overall mass balances are analyzed. Evaluation of the compositional changes in terms of cellulose, hemicellulose and lignin content after pretreatment identifies components which are solubilized during pretreatment in relation to the observed disintegration. Furthermore, we measure changes of acetyl content resulting from acetosolv pretreatment of beech wood for selected samples. Lastly, we evaluate the performed pretreatment experiments based on sugar yields of enzymatic hydrolysis in view of a potential application of acetosolv pretreatment in biorefineries.
2 Materials and methods
2.1 Pretreatment experiments
The employed acetosolv pretreatment liquids consisted of acetic acid (VWR, 100%) with a variety of catalyst acids of different pKa (the organic acids formic acid (Merck, 99%) and oxalic acid (Merck, dihydrate form) as well as the inorganic acids phosphoric acid (Acros organics, 95%, Applichem, 85%), sulfuric acid (Merck, 95%, Applichem, 72%, Carl Roth, 1 molL− 1), hydrochloric acid (Kruse, 30%, Merck, 1 molL− 1, Carl Roth, 37%) and hydroiodic acid (VWR, 57%)). All employed catalyst acids are stronger acids than acetic acid (i.e., lower pKa). The pretreatment liquids were prepared by weight and the water content was not further adjusted but resulted from the purity of the employed catalyst acids (i.e., the water content increased with increasing catalyst molarity). All chemicals were used as received without further purification.
Two main sets of acetosolv pretreatment liquids were investigated. For the first set, pretreatment liquids of all above-mentioned catalysts were prepared at similar molalities to evaluate the influence of the acid strength of the catalyst. These acetosolv experiments were carried out at an approximate ratio of catalyst to acetic acid of 0.25 mmolcat g\(_{\text {AA}}^{-1}\). The second set consisted of acetic acid in combination with hydrochloric, sulfuric or phosphoric acid as three representative catalyst acids to evaluate the influence of varying catalyst molarities (i.e., one type of catalyst at different molar concentrations). The mineral acids sulfuric and hydrochloric acid were chosen because both have been employed for acetosolv pretreatment (cf. [32, 36]). Regarding less strong catalyst acids, the applicability of oxalic acid as a catalyst acid is limited by solubility of oxalic acid in acetic acid–water mixtures [37]. Formic acid is similar to acetic acid and often applied as solvent [29]. Hence, neither formic nor oxalic acid were selected for the experiments with varying catalyst molarities. Regarding strong catalyst acids, hydroiodic acid was also not included in the second set of experiments due to observed light-induced degradation. Hence, we chose phosphoric acid as the third catalyst acid complementing the other two mineral acids.
Additionally, a few experiments with a higher water content of up to 50 mol% were carried out by adding deionized water (inhouse, conductivity approximately 0.8 μS cm− 1) to adjust the water content. Pure acetic acid was used as a reference pretreatment liquid without catalyst to distinguish between the influence of acetic acid as main solvent and catalyst acids. Furthermore, reference experiments containing only water with catalyst acids were carried out to evaluate the interplay of acetic acid and catalyst acid.
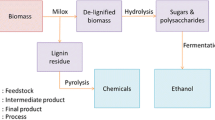
Five hundred milligrams of beech veneer chips (10 mm×2 mm, stored at ambient temperature) at 5 wt% biomass loading was used for all pretreatment experiments (each experiment carried out in duplicate). The veneer had an average moisture content of 4.5 wt%. Experiments were conducted in 50-mL centrifuge tubes that were heated for 2 h in an aluminum heating block at 115 ∘C and atmospheric pressure (typical acetosolv pretreatment conditions, cf. [31, 33, 38]). The samples were stirred at 250 rpm with a magnetic stir bar (15 mm length, 9 mm diameter). Full submersion of the beech chips was checked periodically. After pretreatment, the samples were cooled down with tap water and centrifuged at 10,000 rpm for 10 min. The supernatant was separated for the analysis of the dissolved fraction with low-field NMR. The remaining pretreated, wet biomass was filtered by vacuum with filter crucible POR4 (pore size 10–16 μm) and washed at least 3 times with deionized water. The filter crucibles with the recovered biomass were dried for at least 16 h at 105 ∘C in a drying oven. The main steps of the experiments performed are sketched in Fig. 1. In the following, recovered fraction wr refers to the recovered, dry mass after pretreatment mr as a fraction of the initial amount of beech wood mwood taking into account the moisture content wm of the wood:
The term non-recovered fraction wnr refers to the mass fraction that was not recovered (i.e., solubilized in the pretreatment liquid, not recovered in filter crucible due to small particle size etc.) and is calculated from the closure constraint of wood mass balance:
The recovered fraction was stored at ambient temperature until further analysis (composition, acetylation, enzymatic hydrolysis).
2.2 Analysis of recovered and dissolved fraction
Prior to component analysis and enzymatic hydrolysis, the dried biomass samples were milled in a centrifugal grinding mill (ZM 200 Fritsch, 0.5 mm mesh size).
Acid hydrolysis to determine the composition of native and pretreated beech wood was carried out according to the NREL protocol Determination of Structural Carbohydrates and Lignin in Biomass [39]. Native beech had an average composition of 41.8 wt% cellulose (measured as glucose), 26.2 wt% hemicellulose (measured as xylose and mannose) and 17.3 wt% acid-insoluble lignin.
Enzymatic hydrolysis of pretreated wood samples was carried out in an enzyme–buffer solution containing 14.05 μL mL− 1 Celluclast and 0.1 mol L− 1 sodium acetate buffer at pH 4.8 with a biomass loading of 1 wt% in 1.5-mL Eppendorf tubes. The samples were hydrolyzed in a thermomixer at a stirring rate of 1000 rpm and a temperature of 50 ∘C for 72 h. For the evaluation of hydrolysis effectiveness, the amounts of cellobiose and glucose released during hydrolysis were taken for the calculation of sugar yield from the cellulose fraction.
For the determination of acetyl content, 200 mg of dry wood were added to 10 g of 1 mol L− 1 sodium hydroxide solution and heated at 80 ∘C for 1 h. After heating, the samples were centrifuged at 10,000 rpm for 10 min. A sample of the supernatant was taken for NMR analysis to determine the concentration of acetic acid and further calculate the acetyl content. The solid fraction after deacetylation was filtered and dried in the same manner as the recovered fraction of the pretreated samples.
1H NMR spectra for the analysis of supernatants of pretreatment and deacetylation liquids were recorded on a Magritek Spinsolve Carbon benchtop NMR (42.5 MHz). For each spectrum, 64 scans were collected (6.4 s acquisition time, 15 s repetition time, 90∘ excitation pulse). Phase and baseline correction as well as referencing of peak positions was done with MestReNova software (version 9.1.0 Mestrelab Research S.L.). Peak areas for the quantitative evaluation of components were integrated with PEAXACT (version 4.5 S-PACT GmbH).
3 Results and discussion
For the analysis of acetosolv pretreatment of beech wood, we divide this section according to the scales of the investigated pretreatment phenomena. The first subsection focuses on the macroscopic phenomenon of disintegration and overall mass balances. The second subsection deals with compositional changes after pretreatment, that is, the quantitative analysis of cellulose, hemicellulose and lignin content in the recovered fraction, the qualitative analysis of components that are solubilized during pretreatment and the evaluation of acetyl content of pretreated samples. In the third subsection, pretreatment effectiveness is evaluated by sugar yields of enzymatic hydrolysis.
3.1 Disintegration and mass balances
For a systematic analysis of disintegration, we visually classify the separation of wood fibers after acetosolv pretreatment into five degrees of disintegration (DoD) as shown in Fig. 2. For the allocation of a DoD to a sample, we consider changes in shape of the pretreated wood chips only and do not focus on changes in color. DoD 0 shows no signs of disintegration. It is indistinguishable from untreated wood except for a slight change in color towards a darker brown after pretreatment in some cases. DoD 1 refers to a moderate disintegration. The wood chips are slightly frayed after pretreatment but their original shape is still clearly visible. Furthermore, the amount of disintegrated wood is rather small in comparison to the amount of wood, which is still in original shape. DoD 2 exhibits strong disintegration with largely disintegrated wood. Nevertheless, the form of the original chips is still visible. DoD 3 refers to complete disintegration of the wood. The original shape of the wood chips is no longer visible because fibers have been completely disintegrated from each other. DoD 4 is reached when the fibers are degraded after pretreatment and the wood is charred. In the following, we refer to disintegration or disintegrated wood when a sample reaches DoD 2 or 3 after pretreatment (i.e., DoD 1 is not sufficiently disintegrated, while DoD 4 refers to disintegrated but degraded wood samples).
Classification of degrees of disintegration (DoD) after pretreatment by visual inspection in comparison to the native wood chips (10 mm×2 mm): DoD 0 (no disintegration), DoD 1 (moderate disintegration, slightly frayed wood chips), DoD 2 (strong disintegration, largely disintegrated wood), DoD 3 (complete disintegration) and DoD 4 (charred)
For a first assessment of disintegration after acetosolv pretreatment, experiments with mineral acid catalysts are evaluated, because mineral acids are often employed as catalyst acids in organosolv pretreatment liquids [28]. Figure 3 depicts (a) samples of beech wood pretreated with hydrochloric, sulfuric and phosphoric acid in acetic acid as well as (b) samples of beech wood pretreated with the same mineral acid catalysts in water. With increasing catalyst concentration in acetic acid-based pretreatment liquids, all DoDs are reached with all three mineral acid catalysts. Furthermore, samples of a specific DoD have a similar visual appearance when pretreated with either one of the mineral acid catalysts. Hence, the type of catalyst does not influence the macroscopically visible disintegration. Nevertheless, it is noteworthy that the amounts of catalyst acid required to achieve a certain DoD differ by an order of magnitude from H2SO4 to HCl and from HCl to H3PO4 (see Table 1 for exact values of catalyst acid concentration in acetic acid and water, respectively). Furthermore, with increasing DoD and hence, with increasing catalyst concentration, the samples become darker (cf. [36]).
Beech wood pretreated with mineral acid catalysts a in acetic acid and b in water. Samples are selected to cover the range from no disintegration (DoD 0) to charred samples (DoD 4) after pretreatment in acetic acid-based pretreatment liquids. From top down, catalyst acids are sorted according to increasing pKa. Concentrations of catalyst acids increase from left to right (see Table 1 for values). The position of the vials does not indicate the relative ratios of increasing concentration but results from the size of the vials
While for the acetosolv experiments an increasing DoD up to charred wood is achieved with an increasing catalyst concentration, beech wood does not disintegrate in aqueous pretreatment liquids (i.e., DoD 0). Even at high catalyst concentrations that lead to charring of wood in acetic acid-based liquids (see Table 1), only the edges of the wood chips are charred in aqueous liquids, especially for sulfuric and phosphoric acid catalyst (right samples in Fig. 3 b). This absence of disintegration after pretreatment in aqueous liquids shows that acetic acid plays a major role in achieving disintegration of beech wood during acetosolv pretreatment. Supposedly, all DoDs can be reached irrespective of the type of catalyst acid added to the acetosolv liquids but DoD seems to depend on the acidity (i.e., proton concentration) of the respective pretreatment liquid. For the most part, acids added to glacial acetic acid as solvent remain undissociated, whereas the addition of even small amounts of water can lead to significant increases in the fraction of dissociated acid species [40]. Nevertheless, the relative order of acid dissociation constants in acetic acid appears proportional to the order of these constants in water [41]. Hence, it is of interest to know how the acid strength of a catalyst acid influences the extent of disintegration.
To evaluate the influence of acid strength of catalyst acids in acetosolv liquids on the extent of disintegration, we carried out pretreatment experiments with a variety of catalyst acids. Usually, the acidity of an aqueous solution is indicated by pH. However, a measurement of pH in concentrated, nonaqueous solutions such as the acetosolv liquids requires activity corrections to estimate the amount of dissociated acid species. Because the development of models that account for these influences is not within the scope of this publication, we take the pKa difference between acetic acid and the catalyst acid as an indicator for the acid strength of the catalyst acid in acetosolv pretreatment liquids. This approach has successfully been applied in one study on organosolv pretreatment of Japanese cedar, where sugar yield after pretreatment and enzymatic hydrolysis could be correlated with the pKa of a range of catalyst acids [42].
Figure 4 shows the water content of the pretreatment liquids versus the difference of pKa between acetic acid and the tested catalyst acids for the experiments at constant ratio of catalyst acid to acetic acid. For concentrated acetosolv liquids (filled symbols) below a pKa difference of 5, beech wood does not disintegrate (DoD 0). For an increased pKa difference of 7.76 and 10.76 with sulfuric acid and hydrochloric acid, respectively, the samples are either charred after pretreatment in concentrated acetosolv liquids (filled symbols) or completely disintegrated with added water (striped symbols). Similarly, pretreatment with hydroiodic acid catalyst in acetic acid–water-based pretreatment liquids leads to complete disintegration of beech wood. As stated above, no disintegration is observed for the reference experiments with water and catalyst (checked symbols, see Fig. 3). Hence, the presence of acetic acid is essential to achieve disintegration, while with an increasing water content, the DoD decreases. In addition to acetic acid, a catalyst acid is required to disintegrate the wood because in the experiment with no catalyst (i.e., pure acetic acid as pretreatment liquid) no disintegration was observed.
Water content of pretreatment liquids versus pKa difference between acetic acid and catalyst acid. All catalyst acids are stronger acids than acetic acid. Acetosolv liquids contain approx. 0.25 mmolcatalyst g\(_{\text {AA}}^{-1}\). The filled symbols refer to concentrated acetosolv liquids (i.e., only acetic acid and catalyst acid), where the water content slightly varies due to the different purities of the employed catalyst acids. The striped symbols refer to acetosolv experiments where additional water was added to increase the water content and the checked symbols refer to reference experiments with only water and catalyst acid. The shape of the symbols indicates the DoD (see legend)
Taking the influence of water content into consideration, an evaluation of pKa only does not seem sufficient as a single evaluation criterion for the acid strength of the catalyst acids with regard to disintegration effects. Furthermore, it is unclear how the concentration of the catalyst acids influences pretreatment results. Thus, in the following, three catalyst acids are examined in more detail by studying the influence of their concentration on mass balances and component analysis.
The relation between the non-recovered fraction after pretreatment and the concentration of catalyst acids (a) hydrochloric acid, (b) sulfuric acid and (c) phosphoric acid is sketched in Fig. 5. For all three catalyst acids, the non-recovered fraction of acetic acid-based experiments (filled symbols) increases with increasing concentration. Likewise, a positive correlation between non-recovered fraction and the concentration of hydrochloric acid catalyst has been observed in the literature for acetosolv pretreatment of eucalyptus and beech [33, 38]. Towards higher catalyst acid concentrations, the non-recovered fraction appears limited and remains rather constant.
Non-recovered fraction of beech wood versus concentration of a hydrochloric acid, b sulfuric acid and c phosphoric acid as catalyst acid. Filled symbols refer to liquids with only acetic acid and catalyst acid, striped symbols refer to liquids with water added to acetic acid and catalyst acid and checked symbols refer to reference experiments with only water and catalyst acids. The shape of the symbols indicates the DoD (see legend). Error bars are shown only for measurements with standard deviation above symbol size
With increasing concentration of catalyst acid and hence, increasing non-recovered fraction, the DoD increases successively. Thus, disintegration and non-recovered fraction seem to be interconnected independent of the type of catalyst acid. More specifically, DoD 0 to DoD 2 relate to a non-recovered fraction increasing from slightly more than 0 wt% to approximately 40 wt%, whereas DoD 3 is connected to the maximum recovered fraction in all observed cases. For DoD 4, on the other hand, the non-recovered fraction can decrease due to recondensation of solubilized components. This is visible for sulfuric acid and especially for phosphoric acid.
Furthermore, our experiments show that the range of increasing non-recovered fraction (i.e., the transition from DoD 0 to DoD 2) in relation to catalyst concentration is specific for each catalyst acid. In case of sulfuric acid, a minimum concentration of 1.5–2.9 mmol L− 1 is required to initiate the removal of a significant fraction of wood (i.e., 10 wt% non-recovered fraction). In this concentration range, the pretreated samples show no signs of disintegration (DoD 0). This lower threshold for removal of biomass components is not visible for the other two acids, possibly not resolved due to few data points in the respective concentration ranges. For hydrochloric and phosphoric acid, the lowest tested concentrations with significant removal of biomass components are 0.008 and 0.47 mol L− 1, respectively. Similarly, the transition from increasing non-recovered fraction to the maximum non-recovered fraction is around 0.004–0.0045 mol L− 1, 0.06–0.142 mol L− 1 and 0.9–1.75 mol L− 1 for sulfuric, hydrochloric and phosphoric acid, respectively. However, this order of concentration values does not correlate with the order of pKa, which is pKa,HCl < p\(K_{\text {a,H}_{2}\text {SO}_{4}}\) < p\(K_{\text {a,H}_{3}\text {PO}_{4}}\).
In another study on acetosolv pretreatment of beech wood, sulfuric acid was less effective
as catalyst acid than hydrochloric acid, which matches the order of pKa [36]. In comparison, the pretreatment liquids applied by [36] were not composed of concentrated acetic acid, but contained an increased water content of 20 wt% for both catalysts, while the acetosolv liquids in this study contain only trace amounts of water due to the purity of the acid added as catalyst. Therefore, the water content for hydrochloric acid is slightly higher than for sulfuric acid due to the higher purity of the latter. This comparison to literature implies that the water content in acetosolv liquids, even in trace amounts, influences the overall acidity of the pretreatment liquid and especially the relative order of the strength of catalyst acids. Therefore, a follow-up study with a defined water content would help to resolve the exact relation between disintegration and water content as well as catalyst concentration.
As a first step towards the quantification of the influence of catalyst concentration, the non-recovered fraction is fitted as a function of the catalyst concentration (the dashed lines in Fig. 5 a–c). Often, the solubilization of biomass components during pretreatment is modeled as a first-order reaction [24, 38, 43, 44]. However, biomass is a heterogeneous raw material that shows nonuniform kinetic behavior in many cases (i.e., the assumption of a first-order reaction is not applicable due to changes in reaction parameters during pretreatment or changing reactivities for different fractions of the individual components). To model this varying kinetic behavior, xylan and lignin solubilization during formic acid pretreatment of wheat straw could be related to an extended severity factor in combination with a logistic function [45]. Similarly, a logistic function reflects bulk and residual solubilization of biomass components during formic acid-based pretreatment of sugarcane bagasse [46]. Hence, we chose a logistic function to model the relation between catalyst concentration and non-recovered fraction. Further details on the function and fitted parameters are given in the Supplementary Information. Mainly, the fit supports the observation that the maximum non-recovered fraction is independent of the type of catalyst acid but is specific for beech or for acetosolv-pretreated beech.
As observed above, the presence of water reduces the degree of disintegration. This is also revealed in the analysis of the non-recovered fraction for all three catalyst acids. In aqueous liquids (checked symbols), catalyst acid concentrations that lead to charring of wood in concentrated acetosolv liquids (i.e., DoD 4) only lead to DoD 0 with slightly charred edges (see also Fig. 3). Moreover, for these reference experiments, the non-recovered fraction amounts to approximately 20 wt%, which is lower than the maximum non-recovered fraction for disintegrated samples but higher than the non-recovered fraction for DoD 0 observed with acetic acid-based pretreatment liquids. Similarly, a limited non-recovered fraction of up to 20 wt% was observed for pretreatment of beech wood in aqueous oxalic acid [47].
In experiments with an acetic acid–water mixture as solvent (striped symbols), the non-recovered fraction reaches the upper limit of approximately 40 wt% but with DoD 3 at catalyst concentrations that lead to charring of wood (i.e., DoD 4) in concentrated acetosolv liquids. Hence, besides enabling disintegration, the presence of acetic acid in the pretreatment liquid allows for increased solubilization of removed components. As a result, a rather high maximum non-recovered fraction of 40 wt% is achieved due to disintegration in combination with high solubility for biomass components.
Figure 6 shows the average non-recovered fraction of all experiments with mineral acid catalysts for DoDs 0 to 3 of beech wood after pretreatment (i.e., the average of all experiments with filled symbols shown in Fig. 5). It is visible that also for the average points, the non-recovered fraction increases with increasing DoD (i.e., the recovered fraction is reduced with increasing disintegration). On average, a removed fraction of approximately 30 wt% seems to be the threshold to even slight disintegration (DoD 1). Hence, this limit appears independent of the type of catalyst acid, but similarly to the maximum non-recovered fraction it is unclear, whether this limit is specific for acetosolv-pretreated beech or valid for pretreated beech in general. As another example, in hydrothermal pretreatment of beech wood, a removed fraction of more than 30 wt% resulted from pretreatment severities that also lead to an increased formation of degradation products [48]. These observations indicate that for beech wood, a removal of approximately 30 wt% relates to either formation of degradation products or disintegration for acetosolv and hydrothermal pretreatment, respectively. Therefore, it is of interest to know which factors exactly lead to the disintegration of beech wood. A first step is the component analysis of the recovered fraction to determine whether the removal of certain components correlates with disintegration. Hence, in the next section, we analyze changes in the composition of the samples after pretreatment with the different catalyst acids.
3.2 Compositional changes after pretreatment
First, we quantitatively analyze the pretreated beech wood samples in terms of cellulose, hemicellulose and lignin removed during pretreatment in relation to the overall non-recovered fraction. Second, we qualitatively analyze the components that are dissolved during pretreatment and can thus be determined via NMR spectroscopy of the pretreatment liquids after pretreatment. Third, changes in acetyl content of selected samples after pretreatment are discussed with regard to the influence of the pretreatment liquid composition.
3.2.1 Analysis of recovered material
The fraction of removed components is calculated from the composition and amount of the recovered fraction in comparison to the composition and initial amount of native beech wood.
For pretreatment concepts relying on enzymatic hydrolysis for the release of sugars, it is desirable that a large fraction of cellulose remains in the pretreated sample. This means that glucose is not removed or degraded during pretreatment. For many of our experiments, the standard deviation of removed glucose is high (see Supplementary Information Fig. S1) and therefore, it is unclear how much cellulose actually remains in the pretreated samples. These fluctuations could be caused by an inhomogeneous distribution of sugars in disintegrated samples or by a wrong weight of the recovered fraction due to acetic acid or catalyst acid still being attached to the wood. Furthermore, an underestimation of the glucose content of native beech could lead to negative values of removed glucose. Some experiments show a rather high fraction of glucose removed during pretreatment in combination with a high DoD. Here, aggressive catalyst acids at high concentrations (see Supplementary Information Tab. S2) probably hydrolyze and/or degrade sugars as has been observed for spruce, which was extensively disintegrated after organosolv pretreatment [49]. Nevertheless, it seems that for most experimental conditions, glucose (i.e., cellulose) stays in the recovered fraction.
The sum of removed xylose and mannose as mostly detected sugars of the hemicellulose fraction versus the non-recovered fraction is depicted in Fig. 7 a. In most cases, the standard deviation is low and the graph shows a clear linear correlation between the fraction of hemicellulose sugars removed and the non-recovered fraction. Furthermore, both the samples with concentrated acetic acid-based pretreatment liquids (filled symbols) and the samples with an increased water content in the pretreatment liquids (striped symbols) apparently follow the same correlation. For each catalyst acid, the fraction of removed hemicellulose sugars increases with increasing acid concentration (see Supplementary Information Tab. S2). For the presented experiments, hemicellulose sugars amount to the highest share of the removed components. Nevertheless, especially for the experiments with a high quantity of removed hemicellulose sugars (i.e., complete disintegration with a high non-recovered fraction), other components are removed as well. Moreover, for all (but one) of the disintegrated samples, at least half of the hemicellulose sugars are removed during pretreatment. Thus, the removal of a major fraction of hemicellulose is a prerequisite for disintegration, though this is rather a necessary condition and not sufficient to achieve disintegration.
Fraction of a mannose and xylose, and b lignin removed during pretreatment with acetic acid-based liquids (filled symbols) and acetic acid–water-based liquids (striped symbols) versus non-recovered fraction. The content of hemicellulose as xylose + mannose and the content of lignin in native beech is 26.2 wt% and 17.3 wt%, respectively. The area delineated by the dashed curve highlights experiments with an extraordinary high fraction of lignin remaining in the pretreated material and at the same time a high amount of glucose removed during pretreatment. The shape and color of the symbols indicate the DoD and the employed catalyst acid, respectively (see legend). Error bars are shown only for measurements with standard deviation above symbol size
Figure 7 b shows the fraction of (acid-insoluble) lignin removed during acetosolv pretreatment versus the non-recovered fraction. Generally, lignin has the lowest standard deviations among the quantified components. Similar to hemicellulose, lignin shows a mostly linear correlation between the fraction of lignin removed and the non-recovered fraction, which is in agreement with other studies on organosolv and acetosolv pretreatment [9, 31]. Unlike the removal of hemicellulose sugars, the removal of lignin shows an offset and is only initiated between a non-recovered fraction of 10 to 20 wt%. This means that first a part of hemicelluloses is removed before the lignin content is significantly reduced during pretreatment (i.e., hemicellulose and lignin are removed successively). Similarly, for acetosolv pretreatment of eucalyptus wood, a high delignification of samples is reached when at the same time the pentose concentration in pulping liquors is high (i.e., a large fraction of hemicellulose removed) [38].
For disintegrated samples (DoD 2 and 3), the extent of disintegration in relation to the lignin content is partly ambiguous. More specifically, the visual classification of strong and complete disintegration includes samples with both a high and low fraction of lignin removed. This refers to the samples with an increased water content in the pretreatment liquid (striped symbols) and those that show a rather high amount of glucose removed after pretreatment (highlighted by the dashed curve as in Fig. S1). On the one hand, the strongly acidic conditions which lead to degradation of the cellulose fraction during pretreatment at the same time inhibit an effective removal of lignin. On the other hand, acetic acid–water-based pretreatment allows for an effective removal of lignin with the majority of lignin being removed irrespective of the type of catalyst acid. Thus, in this study, a rather high water content seems to be beneficial to achieve removal of lignin due to a higher solubility for lignin fragments and/or due to a more effective cleavage of lignin in comparison to pretreatment liquids composed of concentrated acetic acid and high catalyst acid concentrations.
Often, estimates for solubility of (bio)polymers in a solvent rely on Hildebrand parameters. According to Hildebrand, solvents allow for solubility of a solute, if their parameters are similar. The Hildebrand parameter value for lignin ranges between 23 and 26 MPa1/2 [50,51,52], while the values for acetic acid and water are determined to 20.7 and 47.9 MPa1/2 (25 ∘C), respectively [53] (note the differently higher reported value for acetic acid in the compilation of [29]). Hence, lignin solubility should be higher in liquids containing a high volume fraction of acetic acid as a result of the volume-based mixing rule to estimate Hildebrand parameters of a mixture (cf. [53]). Correspondingly, solubility of sugarcane bagasse lignin is highest in mixtures containing around 80 vol% acetic acid [34]. Neglecting the rather small density change in acetic acid–water solutions, a volume fraction of around 80 vol% corresponds to a mole fraction of approximately 55 mol%. This is in line with our observation of facilitated lignin removal in pretreatment liquids containing 50 mol% acetic acid in comparison to nearly pure acetic acid.
To conclude, several aspects of the magnitude of recovered material and its composition are linked to disintegration of beech wood after acetosolv pretreatment. For disintegrated samples, the non-recovered fraction exceeds 25 wt%. This corresponds to the removal of approximately one half and one third of the hemicellulose and lignin fraction, respectively. To achieve disintegration in concentrated acetic acid pretreatment liquids, a minimum concentration for each catalyst acid is required: HCl \(\gtrsim \) 0.04 mol L− 1, H2SO4 \(\gtrsim \) 0.005 mol L− 1, H3PO4 \(\gtrsim \) 0.65 mol L− 1 (see Fig. 5). Nevertheless, disintegration also relates to the interplay of acid and water content and hence, this specific threshold of catalyst concentration is probably increased with the addition of water.
3.2.2 Analysis of dissolved components
With the aid of NMR spectroscopy, components that are dissolved in the pretreatment liquid after the experiments can be detected. This analysis of removed components complements the above analysis of the recovered fraction. Sample spectra of aqueous and acetic acid-based pretreatment liquids after pretreatment are given in the Supplementary Information Fig. S2.
Spectra of the reference experiments with water and catalyst show only slight signals of sugars and acetic acid. The latter is probably formed due to deacetylation of beech wood during pretreatment. With the absence of disintegration and hence a high recovered fraction in the reference experiments, it seems plausible that only a few components are dissolved in these pretreatment liquids after pretreatment.
In the spectra of acetosolv pretreatment liquids relying on concentrated acetic acid, dissolved wood components (lignin, sugars) and degradation products (furfural, formic acid) show clear peaks. Both furfural and formic acid are probably formed due to degradation reactions mainly of C5 sugars [43, 54]. Signals of solubilized components and degradation products do not appear similarly for all experiments (see Supplementary Information Tab. S3). Nevertheless, lignin is visible for all experiments with acetic acid-based pretreatment liquids indicating that a certain fraction of the lignin is always extracted independent of the type and concentration of catalyst acid. Besides a very weak lignin signal, the spectra of the reference experiment with only acetic acid and no catalyst acid show no other components. Sugar signals are mostly observed with sulfuric acid catalyst, corresponding to the hydrolysis of cellulose in experiments with sulfuric acid discussed above. In addition to sugar signals, the experiments with sulfuric acid show the strongest signals of degradation products followed by hydrochloric acid and phosphoric acid. This order is in line with the observation that with sulfuric acid, the lowest acid concentrations are required to achieve a certain DoD, whereas for phosphoric acid, the highest concentrations are required (i.e., not in line with the order of pKa). For all three catalyst acids, the furfural peaks are more pronounced the higher the employed acid concentration and vice versa, the formic acid peak is more pronounced the lower the employed catalyst acid concentrations. Furthermore, the spectra of experiments with acetic acid–water-based pretreatment liquids reveal that the addition of water to acetic acid apparently reduces the stability of furfural so that it is further degraded to formic acid (cf. [54]).
3.2.3 Acetyl content
Figure 8 shows the water content of pretreatment liquids versus the acetyl content of pretreated biomass samples. For most experiments, pretreatment liquids contain approximately 0.25 mmolcatalyst g\(_{\text {AA}}^{-1}\) (i.e., the same experiments that were analyzed for the influence of catalyst acid strength in Fig. 4). Since hydrochloric and sulfuric acid catalyst in pure acetic acid lead to undesired charring of wood at this ratio, a lower ratio of 0.06 mmolcatalyst g\(_{\text {AA}}^{-1}\) is chosen for the measurement of acetyl content for these two acids (light and dark blue filled symbols).
Water content of pretreatment liquids versus acetyl content of pretreated biomass samples. Filled symbols refer to liquids with only acetic acid and catalyst acid and striped symbols refer to liquids with water added to acetic acid and catalyst acid. The shape and color of the symbols indicate the DoD and the employed catalyst acid, respectively (see legend). The dashed line indicates the acetyl content measured for native beech wood
The acetyl content of native beech of 4.17 wt% is in accordance with values for beech wood mentioned in the literature, which vary between 3.8 and 4.7 wt% [33, 48, 55]. Except for the hydroiodic acid experiment with a slightly reduced acetyl content, all evaluated experiments exhibit an increase in acetyl content compared to native beech. This means that cellulose and/or lignin is acetylated during pretreatment with the employed acetosolv liquids. Similarly, in other studies, acetylation of either cellulose or lignin has been observed after acetosolv pretreatment of different types of biomass [30, 34, 56]. However, a low acetyl content has been identified as being beneficial for high sugar yields after enzymatic hydrolysis. Unlike low delignification or partial decrystallization of cellulose, the effect of a high acetyl group content cannot be compensated for with increased hydrolysis times [13]. Therefore, a reduction of acetyl content during pretreatment should be aimed at.
For the investigated experiments, visual disintegration does not correlate with acetyl content, because samples with DoD 2 are spread over the range of measured acetyl contents. Nevertheless, the sample with the lowest acetyl content is the only one that is completely disintegrated after pretreatment.
Regarding the composition of the pretreatment liquid, the water content as well as the strength of the catalyst acid influence the acetyl content after pretreatment. It is notable that the acetyl content decreases with decreasing acetic acid content (i.e., increasing water content). Moreover, at similar water contents and comparable catalyst concentration, the application of a stronger catalyst acid (i.e., lower pKa) results in a lower acetyl content of the pretreated sample. Correspondingly, the lignin content in the recovered fraction decreases with increasing water content and catalyst acid concentration. Conversely, pretreatment in concentrated acetosolv liquids with catalyst acids of low concentration or low strength is associated with high acetyl and lignin contents in the recovered fraction. Thus, acetylation of lignin could hinder its removal despite the above discussed high solubility that is expected in these liquids.
3.3 Enzymatic hydrolysis
Generally, high sugar yields after enzymatic hydrolysis are desirable for economic feasibility of lignocellulosic biorefineries. For most experiments though, the sugar yield after hydrolysis is rather low with approximately 5 wt% yield referred to native beech (i.e., in the range of the reference experiment without catalyst, see Supplementary Information Tab. S2). The current set of experiments hence does not suggest an optimal operating point for pretreatment but gives indications for the intrinsic differences of acetosolv pretreatment. Several effects could cause the observed low sugar yields after hydrolysis: the absence of disintegration (i.e., a low increase in surface area), hydrolysis and/or degradation of the cellulose fraction in case of sulfuric acid catalyst (i.e., lower amount of cellulose left for enzymatic hydrolysis), redeposition of solubilized lignin [33, 44, 57] or an increase in acetyl content in comparison to native beech [30, 34, 56].
The two highest sugar yields of 20 to 25 wt% (see Tab. S2) are observed after pretreatment with approximately equimolar acetic acid–water mixtures and hydroiodic or hydrochloric acid catalyst. Both samples are disintegrated after pretreatment. Furthermore, these are two of the three experiments with the highest amount of lignin removed (see Fig. 7) as well as an acetyl content in the range of untreated beech (see Fig. 8). Hence, our findings are in line with observations from the literature that both a low lignin and acetyl content are beneficial for enzymatic hydrolysis. As discussed above, the exact relation between the composition of acetosolv pretreatment liquids (i.e., water content as well as type and concentration of catalyst acid) and the investigated pretreatment phenomena is yet unclear. This requires further research, especially to resolve the influence of a changing water content in the regime of concentrated acetic acid with regard to lignin removal and lignin solubility.
The sugar yields obtained with the above discussed experiments correspond to a cellulose-to-glucose conversion yield of approximately 50 wt%. This yield is clearly higher than yields of acetosolv-pretreated pine and eucalyptus, which are in the range of 10 and 30 to 40 wt%, respectively [56, 57]. However, in comparison to other pretreatment concepts such as alcohol-based organosolv pretreatment with cellulose conversion exceeding 50 wt% [29] or ionic liquids with virtually complete cellulose conversion [22], the researched acetosolv conditions lead to rather low sugar yields and need improvement (e.g., deacetylation step, testing of improved pretreatment liquids). Nevertheless, assuming that the removed hemicellulose sugars can be completely recovered from the pretreatment liquids, the two experiments with the highest glucose yield meet the criterion of 400 g sugars per kg wood for viable biorefinery processes [58].
4 Summary and conclusions
Beech wood was pretreated with a variety of acetosolv liquids composed of acetic acid, different types of catalyst acid and varying water contents as an exemplary pretreatment concept to resolve correlations between pretreatment phenomena and composition of pretreatment liquids. Although, for the tested conditions, the highest sugar yields after enzymatic hydrolysis exceed other reported yields by 10 percentage points and more, the sugar yields are rather at the border of economically viable operation of a biorefinery. Hence, temperature and residence time need improvement for a further increase in sugar yields. More importantly, this study of varying acid and solvent composition enables the analysis of pretreatment phenomena on several scales directly related to the composition of pretreatment liquids. To classify the separation of wood fibers during acetosolv pretreatment, we define five degrees of disintegration from no disintegration to charring of wood. Our experiments show that the degree of disintegration depends on the concentration of catalyst acid and acetic acid. The comparison to aqueous pretreatment liquids reveals that the presence of acetic acid is required to achieve disintegration at all. This is due to an increased catalytic activity of acetic acid in acetosolv liquids containing at least equal amounts of acetic acid and water. In all cases, an increasing concentration of mineral acid catalysts increases the degree of disintegration. Likewise, the non-recovered fraction after pretreatment increases up to a threshold of approximately 40 wt%. This maximum of non-recovered fraction is independent of the type of catalyst acid. Further analysis of the composition reveals that the magnitude of the non-recovered fraction linearly correlates with the amount of both removed hemicellulose and removed lignin. The removal of half of the hemicelluloses together with the removal of one third of lignin is a prerequisite for the disintegration of acetosolv-pretreated beech wood. With regard to the composition of pretreatment liquids, an equimolar ratio of acetic acid and water in combination with a strong catalyst acid allows for a high delignification and prevents an increase in acetyl content, which proves beneficial for enzymatic hydrolysis. To further quantify the relation between composition of pretreatment liquid and pretreatment results, experiments with a defined water content covering the range from concentrated acetosolv liquids with a low amount of water to pretreatment liquids containing an equimolar amount of acetic acid and water would be beneficial. The analysis of pretreatment phenomena shows that disintegration in combination with a non-recovered fraction of around 40 wt% serves as a necessary condition for effective pretreatment. Such a quick and robust analysis of DoD complements a more laborious wet chemical analysis and thus allows for easy screening of pretreatment conditions with regard to a competitive application in biorefineries.
References
Marquardt W, Harwardt A, Hechinger M, Kraemer K, Viell J, Voll A (2010) The biorenewables opportunity – toward next generation process and product systems. AIChE J 56 (9):2228–2235. https://doi.org/10.1002/aic.12380
Regestein L, Klement T, Grande P, Kreyenschulte D, Heyman B, Maßmann T, Eggert A, Sengpiel R, Wang Y, Wierckx N, Blank LM, Spiess A, Leitner W, Bolm C, Wessling M, Jupke A, Rosenbaum M, Büchs J (2018) From beech wood to itaconic acid: case study on biorefinery process integration. Biotechnol Biofuels 11:279. https://doi.org/10.1186/s13068-018-1273-y
Werpy T, Petersen G (2004) Top value added chemicals from biomass: Volume I–results of screening for potential candidates from sugars and synthesis gas
Wyman CE, Dale BE (2015) Producing biofuels via the sugar platform. CEP Magazine 45–57
Luterbacher JS, Rand JM, Alonso DM, Han J, Youngquist JT, Maravelias CT, Pfleger BF, Dumesic JA (2014) Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science (Washington, DC US) 343(6168):277–280. https://doi.org/10.1126/science.1246748
Mosier N, Wyman C, Dale B, Elander R, Lee Y, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Dale BE, Ong RG (2012) Energy, wealth, and human development: Why and how biomass pretreatment research must improve. Biotechnol Prog 28(4):893–898. https://doi.org/10.1002/btpr.1575
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatment technologies. Bioresour Technol 96(18):2019–2025. https://doi.org/10.1016/j.biortech.2005.01.017
Paszner L, Behera NC (1989) Topochemistry of softwood delignification by alkali earth metal salt catalysed organosolv pulping. Holzforschung 43(3):159–168. https://doi.org/10.1515/hfsg.1989.43.3.159
Viell J, Marquardt W (2011) Disintegration and dissolution kinetics of wood chips in ionic liquids. Holzforschung 65(4):519–525. https://doi.org/10.1515/HF.2011.072
Xu J, Zong MH, Fu SY, Li N (2016) Correlation between physicochemical properties and enzymatic digestibility of rice straw pretreated with cholinium ionic liquids. ACS Sustainable Chem Eng 4 (8):4340–4345. https://doi.org/10.1021/acssuschemeng.6b00860
Kong F, Engler CR, Soltes EJ (1992) Effects of cell-wall acetate, xylan backbone, and lignin on enzymatic hydrolysis of aspen wood. Appl Biochem Biotechnol 34(1):23–35
Zhu L, O’Dwyer JP, Chang VS, Granda CB, Holtzapple MT (2008) Structural features affecting biomass enzymatic digestibility. Bioresour Technol 99(9):3817–3828. https://doi.org/10.1016/j.biortech.2007.07.033
Saini JK, Patel AK, Adsul M, Singhania RR (2016) Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew Energy 98:29–42. https://doi.org/10.1515/hfsg.1989.43.3.159
Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS (2010) Cellulose crystallinity – a key predictor of the enzymatic hydrolysis rate. FEBS J 277(6):1571–1582. https://doi.org/10.1111/j.1742-4658.2010.07585.x
Chen X, Shekiro J, Franden MA, Wang W, Zhang M, Kuhn E, Johnson DK, Tucker MP (2012) The impacts of deacetylation prior to dilute acid pretreatment on the bioethanol process. Biotechnol Biofuels 5:8. https://doi.org/10.1186/1754-6834-5-8
Grohmann K, Mitchell DJ, Himmel ME, Dale BE, Schroeder HA (1989) The role of ester groups in resistance of plant cell wall polysaccharides to enzymatic hydrolysis. Appl Biochem Biotechnol 20-21 (1):45–61. https://doi.org/10.1007/BF02936472
Jiang W, Peng H, Li H, Xu J (2014) Effect of acetylation/deacetylation on enzymatic hydrolysis of corn stalk. Biomass Bioenergy 71:294–298. https://doi.org/10.1016/j.biombioe.2014.09.028
Das N, Jena PK, Padhi D, Kumar Mohanty M, Sahoo G (2021) A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Conv Bioref https://doi.org/10.1007/s13399-021-01294-3
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18. https://doi.org/10.1016/j.biortech.2008.05.027
van Osch DJPG, Kollau LJBM, van den Bruinhorst A, Asikainen S, Rocha MAA, Kroon MC (2017) Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys Chem Chem Phys 19(4):2636–2665. https://doi.org/10.1039/c6cp07499e
Viell J, Inouye H, Szekely NK, Frielinghaus H, Marks C, Wang Y, Anders N, Spiess AC, Makowski L (2016) Multi-scale processes of beech wood disintegration and pretreatment with 1-ethyl-3-methylimidazolium acetate/water mixtures. Biotechnol Biofuels 9:7. https://doi.org/10.1186/s13068-015-0422-9
Bär J, Phongpreecha T, Singh SK, Kral Yilmaz M, Foster CE, Crowe JD, Hodge DB (2018) Deconstruction of hybrid poplar to monomeric sugars and aromatics using ethanol organosolv fractionation. Biomass Conv Bioref 8(4):813–824. https://doi.org/10.1007/s13399-018-0330-x
Nitsos C, Rova USL, Christakopoulos P (2018) Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energ (Basel Switz) 11(1):50. https://doi.org/10.3390/en11010050
Myerly RC, Nicholson MD, Katzen R, Taylor JM (1981) The forest refinery. CHEMTECH 11(3):186–192
Nimz HH, Casten R (1986) Chemical processing of lignocellulosics. Holz Roh- Werkst 44 (6):207–212. https://doi.org/10.1007/BF02611993
Schliephake D (1990) Umweltschonende Aufschlußverfahren von pflanzlichem Material zur Gewinnung von Faserzellstoffen. Lenzinger Ber 69:21–28
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82(5):815–827. https://doi.org/10.1007/s00253-009-1883-1
Zhao X, Li S, Wu R, Liu D, Wu R (2017) Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: chemistry, kinetics, and substrate structures. Biofuels, Bioprod Biorefin 11(3):567–590. https://doi.org/10.1002/bbb.1768
Xu F, Sun JX, Sun R, Fowler P, Baird MS (2006) Comparative study of organosolv lignins from wheat straw. Ind Crops Prod 23(2):180–193. https://doi.org/10.1016/j.indcrop.2005.05.008
Ligero P, Vega A, Bao M (2005) Acetosolv delignification of Miscanthus sinensis bark: Influence of process variables. Ind Crops Prod 21(2):235–240. https://doi.org/10.1016/j.indcrop.2004.04.006
Shui T, Feng S, Yuan Z, Kuboki T, Xu CC (2016) Highly efficient organosolv fractionation of cornstalk into cellulose and lignin in organic acids. Bioresour Technol 218:953–961. https://doi.org/10.1016/j.biortech.2016.07.054
Vila C, Santos V, Parajó JC (2000) Optimization of beech wood pulping in catalyzed acetic acid media. Canad J Chem Eng 78(5):964–973. https://doi.org/10.1002/cjce.5450780514
Zhao X, Liu D (2013) Kinetic modeling and mechanisms of acid-catalyzed delignification of sugarcane bagasse by aqueous acetic acid. BioEnergy Res 6(2):436–447. https://doi.org/10.1007/s12155-012-9265-4
Young RA (1998) Acetic acid-based pulping. In: Young RA, Akhtar M (eds) Environmentally friendly technologies for the pulp and paper industry. Wiley, New York, pp 133–156
Kin Z (1990) The acetolysis of beech wood. Tappi J 73(11):237–238
Strassburger J, Torgesen JL (1963) A Phase study of the system: Oxalic acid/acetic acid/water, its significance in oxalic acid crystal growth. J Res Natl Bur Stand Sect A 67A (4):347–350. https://doi.org/10.6028/jres.067A.037
Parajó JC, Alonso JL, Vázquez D (1993) On the behaviour of lignin and hemicelluloses during the acetosolv processing of wood. Bioresour Technol 46(3):233–240. https://doi.org/10.1016/0960-8524(93)90126-V
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (July 2011) Determination of structural carbohydrates and lignin in biomass: Laboratory analytical procedure (LAP). Tech. Rep. NREL/TP-510-42618 National Renewable Energy Laboratory, Golden, Colorado
Smith TL, Elliott JH (1953) Acid–base equilibria in glacial acetic acid. J Am Chem Soc 75 (14):3566–3571. https://doi.org/10.1021/ja01110a075
Hall NF (1931) Acid-base equilibria in non-aqueous solvents with particular reference to glacial acetic acid. Chem Rev 8(2):191–212. https://doi.org/10.1021/cr60030a003
Liu J, Takada R, Karita S, Watanabe T, Honda Y, Watanabe T (2010) Microwave-assisted pretreatment of recalcitrant softwood in aqueous glycerol. Bioresour Technol 101(23):9355–9360. https://doi.org/10.1016/j.biortech.2010.07.023
Fengel D, Wegener G (1989) Wood: Chemistry, ultrastructure reactions. Walter de Gruyter & Co. Berlin, New York
Vázquez G, Antorrena G, González J (1995) Kinetics of acid-catalysed delignification of Eucalyptus globulus wood by acetic acid. Wood Sci Technol 29(4):267–275. https://doi.org/10.1007/BF00202086
Dong L, Wu R, Zhao X, Liu D (2017) Phenomenological modeling and evaluation of formic acid pretreatment of wheat straw with an extended combined severity factor for biomass fractionation and enzymatic saccharification to produce bioethanol. J Taiwan Inst Chem Eng 81:140–149. https://doi.org/10.1016/j.jtice.2017.09.038
Chang X, Zhang J, Wu R, Zhao X (2021) Phenomenological modeling of formic acid fractionation of sugarcane bagasse by integration of operation parameters as an extended combined severity factor. Molecules 26(9):2753. https://doi.org/10.3390/molecules26092753
Fišerová M, Opálená E, Illa A (2013) Comparative study of hemicelluloses extraction from beech and oak wood. Wood Res 58(4):543–554
Nitsos CK, Matis KA, Triantafyllidis KS (2013) Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 6(1):110–122. https://doi.org/10.1002/cssc.201200546
Yawalata D, Paszner L (2006) Characteristics of NAEM salt-catalyzed alcohol organosolv pulping as a biorefinery. Holzforschung 60(3):239–244. https://doi.org/10.1515/HF.2006.039
Balogh DT, Curvelo A, de Groote R (1992) Solvent effects on organosolv lignin from Pinus caribaea hondurensis. Holzforschung 46(4):343–348. https://doi.org/10.1515/hfsg.1992.46.4.343
Schuerch C (1952) The solvent properties of liquids and their relation to the solubility, swelling, isolation and fractionation of lignin. J Am Chem Soc 74(20):5061–5067. https://doi.org/10.1021/ja01140a020
Weerachanchai P, Kwak SK, Lee JM (2014) Effects of solubility properties of solvents and biomass on biomass pretreatment. Bioresour Technol 170:160–166. https://doi.org/10.1016/j.biortech.2014.07.057
Barton AFM (1975) Solubility parameters. Chem Rev 75(6):731–753. https://doi.org/10.1021/cr60298a003
Dunlop AP (1948) Furfural Formation and Behavior. Ind Eng Chem 40(2):204–209. https://doi.org/10.1021/ie50458a006
Yilgor N, Unsal O, Kartal SN (2001) Physical, mechanical, and chemical properties of steamed beech wood. For Prod J 51(11-12):89–93
Pan X, Gilkes N, Saddler JN (2006) Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 60(4):398–401. https://doi.org/10.1515/HF.2006.062
Vázquez G, Antorrena G, González J, Freire S, Crespo I (2000) The influence of acetosolv pulping conditions on the enzymatic hydrolysis of Eucalyptus pulps. Wood Sci Technol 34(4):345–354. https://doi.org/10.1007/s002260000053
Marks C, König A, Mitsos A, Viell J (2020) Minimal viable sugar yield of biomass pretreatment. Biofuels, Bioprod Biorefin 14(2):301–314. https://doi.org/10.1002/bbb.2074
Acknowledgements
We thank Denise Hambsch, Christian Tomala, Frederik Bergs and Pascal Padberg for assistance in conducting the experiments and Christin Cürten for carrying out the enzymatic hydrolysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – Exzellenzcluster 2186 “The Fuel Science Center”. This work was also performed as part of the Cluster of Excellence “Tailor-Made Fuels from Biomass”, which is funded by the Excellence Initiative by the German federal and state governments to promote science and research at German universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1007/s13399-021-02023-6.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marks, C., Viell, J. Acetosolv pretreatment of wood for biorefinery applications. Biomass Conv. Bioref. 13, 11687–11701 (2023). https://doi.org/10.1007/s13399-021-02023-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02023-6