Abstract
The main source of fuel for domestic cooking applications in Sub-Saharan Africa is either locally available firewood species or charcoal produced by slow pyrolysis of these species. However, very few studies exist that characterize and quantify physical properties, burning rates, peak temperatures, and calorific values of typical firewood species and resulting charcoal fuels produced by slow pyrolysis. This study evaluated the mechanical and thermal properties of firewood and charcoal from five tree species namely: Dichrostachys cinerea, Morus Lactea, Piliostigma thonningii, Combretum molle, and Albizia grandibracteata. Characterization was done by scanning electron microscopy, thermogravimetric analysis, bomb calorimetry, Fourier transform infrared spectroscopy, bulk density measurements, and durability, water boiling and absorption tests. SEM images showed the development of macropores on charcoal after slow pyrolysis. Peak temperatures during firewood and charcoal combustion ranged between 515.5–621.8 °C and 741.6–785.9 °C, respectively. Maximum flame temperatures ranged between 786.9–870.8 °C for firewood and 634.4–737.3 °C for charcoal. Bulk densities and calorific values of charcoal species were higher than those for firewood species. Drop strengths for firewood were all 100% while for charcoal were between 93.7 and 100%. Water boiling tests indicated that firewood fuel performed better that charcoal fuel for low amounts of water due to higher maximum flame temperatures obtained during combustion of firewood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Energy is an essential element of economic and societal growth. Energy from biomass has significant potential to have an impact on the developmental challenges of rural poverty and environmental degradation [1,2,3]. Biomass provides about 12–15% of global energy needs [4]. In developing nations, biomass is the main energy source for over 80% of the population [5]. Over 3 billion people rely on the traditional use of solid biomass for cooking and heating purposes [6, 7].
In sub-Saharan Africa, cooking fuels are mainly dominated by firewood in rural areas and charcoal in urban areas [8, 9]. For example, in Uganda, firewood and charcoal production contribute 70.9% of all the forestry activities [10]. Firewood (31.0%–Urban; 85.2% –Rural) and charcoal (58.2%–Urban; 11.8%–Rural) dominate as fuels for domestic cooking applications [11]. Uganda’s forest cover is approximately 2 million hectares, which is estimated at 8.1% of the country’s total land area [10]. Daily consumption of firewood and charcoal stands at 40,500 and 5000 metric tons, respectively [12].
Firewood and charcoal are a major source of income for households in sub-Saharan Africa [13, 14]. The contribution of firewood and charcoal to Uganda’s annual Gross Domestic Product (GDP) is around US$ 48 million and US$ 26.8 million, respectively [10,11,12]. However, this value is limited by the insufficiency in data on firewood and charcoal production. Additionally, limited data availability on properties of particular firewood species and resulting charcoal produced negatively affects forest and energy management policy because knowledge of specific properties can guide policy on which tree species should be grown and harvested for quality firewood and/or charcoal production [12].
Firewood is a porous and fibrous structural tissue obtained from naturally occurring trees. Charcoal is produced from firewood through slow pyrolysis/carbonization [15]. Slow pyrolysis produces a dark residue consisting of carbon (charcoal) and any remaining ash by the slow process of heating firewood in the absence of oxygen [16]. Despite existing knowledge on the thermal conversion of firewood to charcoal by slow pyrolysis, very few studies exist on the properties of local firewood species and resulting charcoal produced [17]. Therefore, the purpose of this study was to perform a comparative analysis of physical, thermal and mechanical properties of five typical local firewood species and resulting charcoal produced from their slow pyrolysis.
2 Materials and methods
2.1 Materials
Firewood from five local commonly used tree species were collected from Luwero District in Central Uganda. Luwero District is located at latitude 0.83°, longitude 32.50° and approximately 75 km from Kampala City. The firewood species collected included: Dichrostachys cinerea (Sickle bush/Kalema Njovu/Muwanika), Morus Lactea (African Mulbery/Mukooge), Piliostigma thonningii (Camel’s foot/Mugaali), Combretum molle (Velvet bush willow/Ndagi), and Albizia grandibracteata (Large-leaved Albizia/Nongo). Firewood species used in this study were obtained from woodlands in central Uganda, which are characterized by moist and dry savanna lands interspersed with bushland and grassland. These woodlands have remained a main source of charcoal for Kampala city given their proximity to the city [13, 18,19,20,21,22].
2.2 Traditional Earth mound “Kasisira” slow pyrolysis thermo-conversion processing
Twenty kilogram portions from each of the collected species was used to make charcoal. Firewood from each tree species were each assembled separately using the “kasisira” method. In this method, firewood is lumped together and then covered with branches and dry leaves for easy ignition before applying layers of damp soil to retain heat during slow pyrolysis (see Fig. 1). Ignited fuel was applied at the top to initiate the slow pyrolysis process [13]. Charcoal formation during pyrolysis is confirmed by reduced smoke and water vapor at the top of the kilns during carbonization of firewood. Slow pyrolysis for firewood carbonization in this study took 48 h.
2.3 Characterization
2.3.1 Scanning Electron Microscopy
Surface morphology and structural changes of the local firewood species and resulting charcoal produced by slow pyrolysis were examined using a Scanning Electron Microscope (SEM) (Vega 3 Tescan). An acceleration voltage of 5 kV was used for all image observations.
2.3.2 Thermogravimetric Analysis (TGA)
An Eltra Thermostep non isothermal Thermogravimetric analyzer, Haan, Germany, was used to obtain physical properties including moisture content, ash content, fixed carbon, and volatile matter of the specific firewood species and charcoal produced by slow pyrolysis of the firewood species [23]. TGA experiments were carried out from room temperature to 920 °C with a heating rate of 16 °C/min. High-purity compressed air (Oxygen:Nitrogen = 21:79, > 99.99%) was used for cleaning the crucibles and chamber prior to TGA experimentation. Nitrogen gas was used as the purge gas for pyrolysis experimentation. The flow rate was maintained at 1 L/min and the sample masses averaged around 1.2 g. Thermogravimetric analysis was used to determine weight loss of the firewood and charcoal samples with increase in temperature [24, 25]. TGA also provided combustion characteristics in terms of differential thermogravimetry (DTG), burning rates and peak temperatures, char residues and mean reactivity of the firewood and charcoal samples. Burning rates were calculated as a ratio of weight change to time taken for the weight to change [26].
2.3.3 Water boiling test
A water boiling test was done in order to evaluate the performance of the specific firewood species and resulting charcoal produced during combustion based on the total time taken to boil 1 L of water on local improved stove using 200 g of fuel [17, 27]. The flame after boiling the water was observed and the maximum attainable temperature of the samples at this point was recorded using a DT-8865 noncontact infrared thermometer gun (Dual laser up to 1000 °C; 30:1 D/S ratio) [28].
2.3.4 Calorific value
Calorific values of specific firewood species and resulting charcoal produced after slow pyrolysis carbonization were determined using an IKA C2000 Oxygen bomb calorimeter [27]. Firewood and charcoal samples were compressed into small blocks weighing about 0.8 g. The measured blocks were fed into the bomb with a wick for transferring flame. Combustion was initiated using pure oxygen (99.95%) at about 30 bars. The blocks ignited electrically within the ignition device and the increase in temperature of the water in the inner vessel of the bomb calorimeter was measured resulting into determination of the calorific values [24].
2.3.5 Fourier Transform Infrared (FTIR) spectroscopy
The FTIR spectra for each firewood species and resulting charcoal samples produced were collected in the range of 4000 to 400 cm−1 using a Jasco FT/IR-6600 type A machine. The resolution was 4 cm−1. Scanning speed was 2 mm/sec. Firewood and charcoal samples were ground to obtain very fine powders using a mortar and pestle. The aim of this test is to identify functional groups in the firewood and charcoal based on FTIR spectra [23].
2.3.6 Bulk density determination
Bulk density of specific firewood species and resulting charcoal produced by slow pyrolysis were determined using a mass and volume ratio analysis. The ratio of mass of a unified material rigidly compressed to the volume of a standard cylinder whose volume is known was used [24, 29].
2.3.7 Durability tests
Drop test method was used to determine the compaction integrity of the firewood and charcoal. Firewood and charcoal samples were elevated up to 2 m and then dropped onto a thick steel plate. The ratio of the weight after dropping to the weight before dropping was recorded as the drop strength. Axial compression test involved crushing firewood samples placed between flat surfaces of a Universal testing machine (10 mm/min test speed), until its structure failed. Drop and compressive strengths are a measure of the extent to which biomass fuels retain their form during packaging, storage and transportation [27, 28, 30].
2.3.8 Water absorption tests
Samples of firewood and charcoal were each soaked in 10 L of water at room temperature for a period between 1 and 5 days. Their weight was measured prior to and after immersion in water. Water absorption was obtained as a percentage of weight increase to the original weight [31].
3 Results and discussion
3.1 Surface morphology
SEM images for local firewood species are shown in Fig. 2(a-e). SEM images after slow pyrolysis to produce charcoal from the local firewood species are shown in Fig. 2(f-j). The fibrous nature of local firewood species can be clearly seen prior to pyrolysis taking place [32]. Slow pyrolysis process damages the original surface and alters the structure of the local firewood species. The produced charcoal is characterized by the presence of macropores of different diameters specific to actual firewood species. These macropores are generated as a result of devolatilization resulting in the formation of a structured carbon network and rudimentary pore structure [32, 33]. Highest pore density was observed for charcoal produced from the slow pyrolysis of Dichrostachys Cinerea firewood species. Charcoal produced from slow pyrolysis of Morus Lactea firewood species showed the lowest pore density with larger rectangular carbon networks observed.
3.2 Physical properties
Physical properties including moisture content, volatile matter, ash content, and fixed carbon for each firewood species and resulting charcoal produced by slow pyrolysis are shown in Table 1. Moisture contents for firewood and resulting charcoal produced ranged between 16.6 – 41.3% and 5.8 – 8.9%, respectively. Morus Lactea firewood species had the highest moisture content. Interestingly after slow pyrolysis to produce charcoal Morus Lactea had the lowest moisture content. The least moisture content among the firewood species of 16.6% was obtained in Dichrostachys cinerea species while the highest charcoal moisture content of 8.9% was obtained after slow pyrolysis of Piliostigma thonningii firewood species. Similar results of moisture content for charcoal were obtained by Briseño-Uribe et al., 2016 (4.9 – 9.7%) and Júnior et al., 2020 (8.5%) [34, 35]. Moisture content is an extremely important property because it has a direct effect on the burning characteristics and calorific values of biomass fuels since high moisture content in firewood implies more energy is used in latent evaporation of water prior to actual fuel combustion for energy production [36, 37]. The moisture content results for charcoal produced from all firewood species except for Piliostigma thonningii firewood species conform to European standard EN 1860–20 which states that ash content should not exceed 8% (EN 1860–2) [38]
Volatile matter ranged between 45.7 – 63.9% and 9.8 – 16.9% for firewood and resulting charcoal produced, respectively. Charcoal has less volatile matter than firewood as most of the volatiles are given off during pyrolysis of firewood to form charcoal [34, 37]. Dichrostachys cinerea firewood and charcoal had the highest and lowest volatile matter composition, respectively. Similarly, Morus Lactea firewood and resulting charcoal produced had the lowest and highest volatile matter contents, respectively. These results are supported by the SEM results shown in Fig. 2, which showed higher macropore density due to devolatilization for Dichrostachys cinerea charcoal and lowest macropore density for Morus Lactea charcoal. Findings for volatiles obtained in this study are within the range obtained by Briseño-Uribe et al., 2016 for charcoal (3.2 – 35%) [35]. Volatiles are released between 190 \(^\circ\) C and 450 \(^\circ\) C during slow pyrolysis due to the presence of hemicellulose, cellulose, and lignin in the biomass biochemical structure [39,40,41]. Low volatile matter compositions for charcoal imply cleaner combustion process with lower smoke emissions and low tar residues produced during domestic cooking applications. This has a positive health dimension [35, 37]. However, higher volatiles enhance fuel ignitability and combustion due to increased chemical reactivity [28, 36].
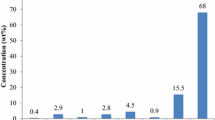
Ash contents of specific firewood species and the resulting charcoal produced ranged between 0.6 – 1.2% and 3.2 – 28.3%, respectively. Dichrostachys cinerea firewood yielded the least ash content (0.6%) while the highest ash (1.15%) was realized in Combretum molle species. Meanwhile, for resulting charcoal produced by slow pyrolysis of the firewood species, Piliostigma thonningii and Combretum molle species had the highest and lowest ash contents, respectively. Generally, firewood had lower ash contents compared to charcoal. Ash content values in this study are similar to those obtained by Ruiz-Aquino et al., 2015, for firewood (0.30–1.00%) but much lower than ash for the charcoal used in the same study (1.0–2.7%) [37]. Mitchual et al., 2014, reported ash contents ranging between 0.61 and 5.04% for firewood [42]. Briseño-Uribe et al., 2016 obtained lower ash contents for charcoal (2.0–8.2%) [35]. Júnior et al., 2020 reported lower ash contents (6.6%) for charcoal [34].
Higher ash contents in charcoal compared to firewood are due to water and volatile matter releases as pyrolysis takes place, leaving a large part of the ash in the solid fuel, which implies that ash content must inevitably increase with increasing temperature and carbonization duration [43]. Experimental studies on torrefaction of Dichrostachys cinerea wood showed that ash content increased from 6.81% to 7.81% when torrefaction time and temperature increased from 60 min and 250 °C to 120 min and 290 °C, which implies that higher ash content results should be expected in kilns where slow pyrolysis duration and temperature are increased [44]. For charcoal produced from Dichrostachys cinerea and Piliostigma thonningii species, the effect of higher charring temperature and the fact that slow pyrolysis duration of 48 h was considered affected their morphological characteristics where cell-wall thinning and volumetric shrinkage were observed as shown in Fig. 2(f) and (g) [45]. Additionally, Dichrostachys cinerea grows in low quality clay or sandy soils and is known to have higher ash content when compared to other types of woody biomass [46]. Ash content for charcoal produced from Dichrostachys cinerea and Piliostigma thonningii firewood species are higher than the limit of 8% in the European standard EN 1860–2 [38]. Ash content significantly influences the rate at which heat is transferred to the surface of a given fuel as well as the diffusion of oxygen to the fuel surface during char combustion [47]. Therefore, high ash content leads to reductions in calorific values of biomass fuels, which is disadvantageous for heating and domestic cooking applications [27, 28].
Results from Table 1 generally indicate that firewood had lower fixed carbon compositions than charcoal. The compositions ranged between 12.1–20.3% and 53.0–74.2% for firewood and charcoal, respectively. Among the firewood species, Morus Lactea had the least fixed carbon composition (12.1%), followed by Albizia grandibracteata (15.0%), Combretum molle (17.3%), Piliostigma thonningii (17.4%), and Dichrostachys cinerea had the highest fixed carbon composition (20.3%). Slow pyrolysis process reduced the amount of water and volatiles in the firewood resulting in an increase in the fixed carbon content in the developed charcoal [43]; Maximum fixed carbon in charcoal was obtained in Albizia grandibracteata species while least fixed carbon compositions in charcoal were obtained in Piliostigma thonningii species. The high fixed carbon content in charcoal compared to firewood is a direct result of the devolatilization process during pyrolysis and hence the decrease in volatiles [43]. Fixed carbon is directly related to calorific value as higher fixed carbons in biomass fuels have been noted to indicate higher calorific values [9]. This is because fixed carbon decreases the residence stage until combustion is completed. Therefore, as volatile matter increases, the percentage of fixed carbon decreases and vice versa [48].
3.3 Thermal properties
3.3.1 Weight loss and DTG
Thermograms for weight loss and DTG results for firewood and charcoal are shown in Fig. 3. Figure 3a shows the weight loss behavior for firewood resulting from increasing the temperature between room temperature and 1000 °C. Weight loss is maintained from combustion at room temperature to about 104 °C, followed by undergoing a major weight loss at about 105 °C. This weight loss occurred due to the evaporation of moisture from the firewood species. After this temperature, the weight remains almost constant up to about 300 °C, where the thermal decomposition of the chemical components in firewood begins. Exothermic reactions start, and combustion occurs in parts where hydrocarbons with a low boiling point are exposed [35, 49]. Burning of the firewood intensifies in the range of 300–600 °C and continues to approximately 800 °C but at lower intensity. When the temperature rises above 800 °C, lignin decomposes, and the remaining weight percentage is mainly composed of residues, including, ash, tars, and fixed carbon [9].
DTG curves show two characteristic stages of thermal decomposition of firewood during combustion (see Fig. 3b). At 105 °C, a major decomposition rate is observed, followed by increases in weight degradation with time. DTG thermograms show the decomposition maximums in single peaks owing to the degradation of cellulose [50]. The point of highest intensity corresponds to peak temperature at which the respective reaction occurs most dominantly. Peak temperatures for firewood species ranged between 515.4 °C and 621.7 °C with Combretum molle firewood and Dichrostachys cinerea firewood species achieving the lowest and highest peaks, respectively. After the peak temperatures of the different firewood species are reached, decomposition proceeds at a more pronounced manner until 800 °C shown by the steep increase in DTG after the peak temperature (see Fig. 3b). Between 800 °C and about 920 °C, change in weight with time is minimal owing to the high molecular weight structure of lignin which accounts for about 30% of firewood that is decomposed off at high temperatures [51].
TGA graphs for charcoal produced by slow pyrolysis for each firewood species are shown in Fig. 3c. Unlike results for firewood species shown in Fig. 3a, charcoal degrades through two main stages. Weight loss is maintained from combustion at room temperature to about 104 °C, followed by a major weight loss at about 105 °C. Loss in weight at this temperature is more pronounced in Piliostigma thonningii charcoal species at 8.9% and least pronounced in Morus Lactea charcoal species (5.8%). These steep losses are explained by the moisture contents of the respective charcoal species. Piliostigma thonningii has the highest moisture content and Morus Lactea has the lowest moisture content (See Table 1). After 105 ºC, the weight remains almost constant up to about 400 °C, where the thermal decomposition of the chemical components in charcoal begins. Burning of charcoal intensifies in the range of 400–920 °C at which char residues are left. These char residues range between 78.1% and 82.9% with Morus Lactea charcoal and Albizia grandibracteata charcoal having the least and most char residues, respectively. Char residues for charcoal are higher than for firewood species because of the higher fixed carbon compositions in the pyrolyzed charcoal produced (see Table 1).
Figure 3d shows DTG curves for the charcoal species. There are two main degradation peaks corresponding to thermal decomposition of charcoal during combustion. The first peak exists between 104 and 105 °C where between 17.0% and 25.9% of the original weight in the charcoal species is lost due to moisture loss. At this temperature range, a maximum decrease in weight per unit time is observed in Piliostigma thonningii charcoal species (0.35%/min) at 104.1 °C. After this temperature range, low changes in weight per unit time are noticed. Peak temperatures occur at a second peak owing to the devolatilization of cellulose materials [52]. Such a two peak behavior is common among materials with a high carbon content [53]. Peak temperatures for charcoal species ranged between 741.6 °C and 785.9 °C with Dichrostachys cinerea and Piliostigma thonningii charcoal species achieving the lowest and highest peaks respectively. After the peak temperatures of the different charcoal species are reached, the change in weight as time increases starts to rise until the final decomposition temperature (920 °C).
3.3.2 Burning rates
Burning rates for firewood are shown in Fig. 4a. Burning rates increased from the beginning of the test, maintained high levels until 50–75 min (depending on the firewood species), and then decreased until about 185 min. After this time, very sharp increases were noted until each charcoal species reached a maximum burning rate value. Highest burning rate during combustion of firewood at this stage was in Dichrostachys cinerea species at 191.8 min (0.014959 g/min). Because of its highest burning, Dichrostachys cinerea obtained the highest peak temperature of 621.8 °C (see Table 2). Piliostigma thonningii species had a lower increase, reaching 0.007398 g/min at 194.1 min.
For charcoal species, burning rate peaks were noted in the first 18 min. Increased burning rates proceeded after that until 37–49 min depending on the charcoal species. At 37 min, burning rate of 0.001348 g/min was obtained in Morus Lactea charcoal while at 49 min, burning rate of 0.002766 g/min was obtained in Piliostigma thonningii charcoal. After this, burning rates decreased to minimum values at 181.0, 109.8, 176.6, 174.4, and 111.2 min for Dichrostachys cinerea (0.000594 g/min), Morus Lactea (0.000676 g/min), Piliostigma thonningii (0.001046 g/min), Combretum molle (0.000406 g/min), and Albizia grandibracteata (0.00086 g/min) charcoal, respectively. Morus Lactea and Albizia grandibracteata charcoal burning rates reached minimum values earlier than other species. This can be attributed to the fact that they have the lowest moisture contents (5.78% for Morus Lactea and 7.01% for Albizia grandibracteata) (see Table 1).
3.3.3 Peak temperatures
Peak temperatures of firewood and charcoal ranged between 515.5–621.8 °C and 741.6–785.9 °C, respectively (see Table 2). Among the firewood species, Combretum molle had the lowest peak temperature (515.5 °C), followed by Albizia grandibracteata (540.9 °C), Piliostigma thonningii (554.4 °C), Morus Lactea (570.22 °C) while Dichrostachys cinerea had the highest peak temperature (621.75 °C). For charcoal species, the lowest peak temperature was realized in Dichrostachys cinerea (741.6 °C), followed by Morus Lactea (755.57 °C), Combretum molle (780.5 °C) and Albizia grandibracteata (785.7 °C). Piliostigma thonningii charcoal had the highest peak temperature (785.9 °C). Similarity in peak temperatures of Albizia grandibracteata charcoal and Piliostigma thonningii charcoal is possibly due to the very minimal variation in their char residues. Firewood species had lower peak temperatures compared to the resulting charcoal produced after slow pyrolysis of the specific firewood species. Lower peak temperatures in the firewood species were due to the fact that firewood burns for less time compared to charcoal implying a lesser aggressive carbonization process with limited removal of volatile matter and secondary reactions between the solid and the gases in vapor phase [54]. Additionally, increased porosity is related to an increase in peak temperature due to higher extraction of volatiles [55].
3.3.4 Boiling characteristics
The time taken to boil 1 L of water ranged between 5–6 min for firewood and 8–10 min for charcoal (See Table 2). Among the firewood species, Morus Lactea and Piliostigma thonningii took the least time to boil 1 L of water (5 min) while Dichrostachys cinerea, Combretum molle and Albizia grandibracteata each took 6 min. For charcoal species, Morus Lactea took 8 min, followed by Piliostigma thonningii and Combretum molle (9 min). Dichrostachys cinerea and Albizia grandibracteata charcoal species each took the most time to boil 1 L of water (10 min). Time differences between the firewood and charcoal species was attributed to the significant differences in their physical property characteristics (see Table 1). Firewood took less time because it has less ash compositions. Low boiling times are explained by high volatile matter compositions in non-carbonized fuels (firewood species) compared to the carbonized fuels (charcoal species) [17, 28]. Maximum flame temperatures reached during the water boiling ranged between 786.9–870.8 °C for firewood and 634.4–737.3 °C for the resulting charcoal produced (see Table 2). Firewood as an energy fuel boiled water in less time than charcoal because maximum flame temperatures reached were higher for firewood than charcoal. Charcoal has higher peak temperatures, and therefore, it takes a longer time to get to the point of maximum weight loss and this delays the time at which maximum flame temperature can be given off during combustion (see Table 2).
3.3.5 Calorific values for firewood species and produced charcoal
Calorific values ranged between 11.3–16.7 MJ/kg and 28.1–30 MJ/kg for specific firewood species and charcoal produced by slow pyrolysis of each specific firewood species, respectively (see Table 2). Among the firewood species, Morus Lactea had the lowest calorific value (11.3 MJ/kg), followed by Albizia grandibracteata (15.1 MJ/kg), Combretum molle (15.6 MJ/kg), Piliostigma thonningii (15.9 MJ/kg) while Dichrostachys cinerea had the highest calorific value (16.7 MJ/kg). For charcoal species, the lowest calorific value was observed for Dichrostachys cinerea (28.1 MJ/kg), followed by Albizia grandibracteata (28.4 MJ/kg), Morus Lactea (28.6 MJ/kg) and Combretum molle (28.9 MJ/kg). Piliostigma thonningii charcoal had the highest calorific value (30 MJ/kg). The calorific values of charcoal obtained in this study are slightly lower than those obtained by Ruiz-Aquino et al., 2015, (32.00–33.30 MJ/kg), but within the range reported by Briseño-Uribe et al., 2016, (26.5–31.4 MJ/kg) [35, 37]. Charcoal achieved higher calorific values than firewood because charcoal contains more fixed carbon than firewood as a result of the carbonization process where moisture and volatile matter are expelled from the firewood by the thermo-chemical carbonization process during slow pyrolysis. Fixed carbon is known to have a direct relationship with calorific value [9, 30, 43]. Calorific value also gives an indication of the amount of heat and the potential value of electricity that can be produced by the biomass fuel [56, 57].
3.4 FTIR analysis
FTIR spectra shown in Fig. 5a and b show that the underlying chemical structure of the firewood species and charcoal produced by slow pyrolysis of these species is very similar as spectrograms do not show significant differences as the tree species used for producing charcoal are the same firewood species. Different studies have also shown similar spectra for firewood species and charcoal [23, 58]. Bands observed between 3900 and 3500 cm−1 are associated with O − H vibrations in hydroxyl groups due to the presence of phenolic groups [59]. At this wavelength interval, moisture content could take part in the formation of hydrogen bonds [60]. The O − H stretching band presents similar shape and intensity between the firewood species (see Fig. 5a) as well as between charcoal species (see Fig. 5b). The weak peak at around 2920 cm−1 is assigned to the stretching vibration of aliphatic C − H, which suggests the presence of cellulose and hemicellulose components in the bio-chemical structure [61, 62]. Characteristic transmittance bands at 1900–2400 cm−1 are due to CO2 [63]. The peak at around 1505 cm−1 corresponds to aromatic C = C bond stretching in lignin. The peak at around 1000 cm−1 represents the C–O–C stretching in ether bonds of lignin [64]. The peak obtained at wavelengths below 500 cm−1 suggested the C-X stretching vibration of Halocarbon compounds [63].
3.5 Mechanical properties
3.5.1 Bulk density results
Bulk density of specific firewood species and charcoal produced by slow pyrolysis ranged from 481 to 537.6 kg/m3 and 615.5 to 707.4 kg/m3, respectively (see Fig. 6a). Since the firewood species had relatively high bulk densities, it is not surprising that the charcoal obtained from these firewood species had even higher bulk densities [35, 37, 55]. Densities reported for wood are in line with average values of 500 kg/m3 reported as the minimum value for charcoal production. The minimum bulk density for good quality charcoal has been reported as 400 kg/m3, which implies that all of the charcoal produced in this study qualifies as a quality charcoal according to that criterion [65, 66]. The higher densities for charcoal are attributed to the effect of temperature and compression during the pyrolysis process. Somerville and Jahanshahi (2015) reported apparent densities of charcoal of about 1000 kg/m3 at 0.5 MPa between 400 and 600 °C as a result of an increase in charcoal porosity at higher pyrolysis temperatures for Australian eucalypt wood [67]. Combretum molle had the highest bulk density among the firewood species while charcoal produced by slow pyrolysis of Piliostigma thonningii firewood species had the highest bulk density among the charcoal variants in the study, further suggesting its higher likelihood of having higher energy per unit volume [68]. During slow pyrolysis of firewood, most of the voids escape from the structure of firewood as volatiles creating a brittle network of macropores (see Fig. 2). Bulk density of a fuel is important for domestic cooking or heating purposes. Denser fuels are more desired because they burn for longer times, therefore reducing the amount of fuel used for domestic cooking or heating purposes [68, 69].
3.5.2 Drop strength and compressive strength
Drop strength results for firewood and charcoal are shown in Fig. 6b. The drop strengths of firewood were all 100% while drop strengths for charcoal ranged between 93.7% and 100%. Compressive strengths for firewood species ranged between 4.1 and 21.3 MPa (see Table 3). Higher compressive strength and drop strength results are advantageous for transportation and storage of biomass fuels [27, 28, 35]. These compressive strength results are related to bulk density results for these firewood species [70]. Stress–strain curves showed longer linear elasticity for Dichrostachys cinerea, followed by Combretum molle, Piliostigma thonningii, Albizia grandibracteata and finally, Morus Lactea (see Fig. 7). Bulk density of wood has a positively correlation with its compressive strength [71]. These results also indicate that weight loss is also correlated with compressive strength (see Fig. 3). Dichrostachys cinerea firewood had a recorded weight loss of (78.2%) while Morus Lactea had the highest weight loss (86.2%).
3.6 Water absorption results
As shown in Fig. 8a, the water absorption of firewood species increased with increase in immersion time. Highest water absorption rates were observed within the first 24 h of immersion where between 17.7% and 32.86% increases were recorded. Increases were lowest in the Dichrostachys cinerea species and highest for Piliostigma thonningii species. From 96 h immersion time, water absorption capability of firewood species plateaued and percentage increases between 96 and 120 h were 1.7%, 2.7%, 1.4%, 1.2%, and 3.4% for Dichrostachys cinerea, Morus Lactea, Piliostigma thonningii, Combretum molle, and Albizia grandibracteata species, respectively [72].
Results for water absorption by charcoal are shown in Fig. 8b. Increases in water absorption after 24 h were 40.1%, 42.1%, 50.0%, 50.0%, and 57.8% for Dichrostachys cinerea, Morus Lactea, Piliostigma thonningii, Combretum molle, and Albizia grandibracteata species, respectively. After 24 h, water absorption in all species apart from Combretum molle continues to rise until the end of day 3 (72 h) before gradually reducing for the remaining immersion time. After 72 h, Morus Lactea charcoal species had 120% water absorption rates. For Combretum molle charcoal, water absorption ability reduced between 24 and 48 h before gradually increasing to a maximum (120%) at the end of day 4 (96 h). Further immersion for an additional 24 h, decreased Combretum molle’s water absorption capability to 53.3%. Compared to firewood species, charcoal takes less immersion time to reach maximum water absorption levels due to its higher specific area due to the presence of macropores on the charcoal surface (see Fig. 1). The difference in water absorption behavior among the respective species of firewood and charcoal can be explained by differences in the physical structure and morphology between firewood and charcoal as a result of slow pyrolysis where porosity of charcoal is initiated and propagated as a result of devolatilization (see Fig. 2). Final water absorption results at 120 h correspond very well with macropore intensity with Dichrostachys cinerea maintaining more water absorption potential compared to Morus Lactea which was characterized by low macropore intensity (see Fig. 2).
4 Conclusions
In this study, specific firewood species namely: Dichrostachys cinerea, Morus Lactea, Piliostigma thonningii, Combretum molle, and Albizia grandibracteata and resulting charcoal produced by slow pyrolysis of the firewood species were characterized to determine their thermal and mechanical properties. The process of carbonization during slow pyrolysis of firewood in production of charcoal was responsible for reductions in moisture content and volatile matter and an increase in ash content and fixed carbon observed in resulting charcoal produced for each specific firewood species. SEM images showed the development of macropores on charcoal due to devolatilization. Higher calorific values were obtained for charcoal produced by slow pyrolysis of the firewood species. Firewood and charcoal thermal degradations were notably different with peak temperatures of specific firewood species and resulting charcoal ranging from 515.5 \(^\circ\) C to 621.8 °C and 741.6 \(^\circ\) C to 785.9 °C, respectively. Maximum flame temperatures reached during the water boiling test ranged from 786.9 to 870.8 °C for the firewood species and 634.4 to 737.3 °C for produced charcoal. Firewood took less time to boil water due to the higher maximum attainable flame temperatures during firewood combustion. Drop strengths for all firewood species were 100% while drop strength for charcoal varied between 93.7 and 100%. Compressive strength for specific firewood species ranged between 4.1 and 21.3 MPa. Water absorption in firewood species increased with increase in immersion time but at very low variations. Water absorption behavior for charcoal was related to charcoal’s macropore intensity and sites available for absorption.
References
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Biores Technol 83(1):37–46
Karmaker SC, Rahman MM, Hosan S, Saha BB (2020) The impact of biomass energy consumption on human development: evidence from Asian countries. Sciences (IEICES) 6:204–211
Samadi SH, Ghobadian B, Nosrati M (2020) Prediction and estimation of biomass energy from agricultural residues using air gasification technology in Iran. Renewable Energy 149:1077–1091
REN21, R. (2020). Global Status Report, 2020
Neina D, Faust S, Joergensen RG (2020) Characterization of charcoal and firewood ash for use in African peri-urban agriculture. Chem Biol Technol Agric 7:5. https://doi.org/10.1186/s40538-019-0171-2
Sana A, Kafando B, Dramaix M, Meda N, Bouland C (2020) Household energy choice for domestic cooking: distribution and factors influencing cooking fuel preference in Ouagadougou. Environ Sci Pollut Res 27:18902–18910
Del Rio DDF, Lambe F, Roe J, Matin N, Makuch KE, Osborne M (2020) Do we need better behaved cooks? Reviewing behavioural change strategies for improving the sustainability and effectiveness of cookstove programs. Energy Res Soc Sci 70:101788
Bamwesigye D, Kupec P, Chekuimo G, Pavlis J, Asamoah O, Darkwah SA, Hlaváčková P (2020) Charcoal and wood biomass utilization in Uganda: the socioeconomic and environmental dynamics and implications. Sustainability 12(20):8337
Lubwama M, Yiga VA, Muhairwe F, Kihedu J (2020) Physical and combustion properties of agricultural residue bio-char bio-composite briquettes as sustainable domestic energy sources. Renew Energy 148:1002–1016
Uganda Bureau of Statistics (UBOS) (2020) Statistical abstract. Kampala, Uganda
Uganda Bureau of Statistics (UBOS), The National Population and Housing Census 2014-Main Report (Kampala, Uganda), 2016
MEMD (Ministry of Energy and Mineral Development) (2016) National Charcoal Survey for Uganda 2015. Final Report. Government of Uganda
Nabukalu C, Gieré R (2019) Charcoal as an Energy Resource: Global Trade, Production and Socioeconomic Practices Observed in Uganda. Resources 8(4):183
Branch A, Martiniello G (2018) Charcoal power: the political violence of non-fossil fuel in Uganda. Geoforum 97:242–252
Hammerton J, Joshi LR, Ross AB, Pariyar B, Lovett JC, Shrestha KK, Gasson PE (2018) Characterisation of biomass resources in Nepal and assessment of potential for increased charcoal production. J Environ Manage 223:358–370
Ekhuemelo DO, Tsembe JI, Amonum JI (2017) Investigation of charcoal production in Gwer west and Gwer east local government areas of Benue State, Nigeria. Asian J Environ Sci 3:1–13. https://doi.org/10.9734/AJEE/2017/34362
Tumutegyereize P, Mugenyi R, Ketlogetswe C, Gandure J (2016) A comparative performance analysis of carbonized briquettes and charcoal fuels in Kampala-urban, Uganda. Energy Sustain Dev 31:91–96
Namaalwa J, Hofstad O, Sankhayan PL (2009) Achieving sustainable charcoal supply from woodlands to urban consumers in Kampala, Uganda. Int For Rev 11(1):64–78
Musinguzi WB, Okure MAE, Wang L, Sebbit A, Lovas T (2012) Thermal characterization of Uganda’s Acacia hockii, Combretum molle, Eucalyptus grandis and Terminalia glaucescens for gasification. Biomass Bioenergy 46:402–408
Ojelel S, Otiti T, Mugisha S (2015) Fuel value indices of selected woodfuel species used in Masindi and Nebbi districts of Uganda. Energy Sustain Soc 5:14
Kiyinji I, Kidiya JM, Gwali S, Okullo P, Byabashaija DM (2010) Tree species composition, structure and utilization in Maruzi hills forest reserve in Uganda. South For 72(2):113–117
Kalema VN, Witkowski ETF, Erasmus BFN, Mwavu EN (2015) The impacts of changes in land use on woodlands in an equatorial African savanna. Land Degrad Dev 26:632–641
Díez HE, Pérez JF (2017) Physicochemical characterization of representative firewood species used for cooking in some Colombian regions. Int J Chem Eng 2017:4531686. https://doi.org/10.1155/2017/4531686
de Paula Protásio T, Scatolino MV, de Araújo ACC, de Oliveira AFCF, de Figueiredo ICR, de Assis MR, Trugilho PF (2019) Assessing proximate composition, extractive concentration, and lignin quality to determine appropriate parameters for selection of superior Eucalyptus firewood. BioEnergy Research 12(3):626–641
de Paula Protásio T, Bufalino L, Tonoli GHD, Junior MG, Trugilho PF, Mendes LM (2013) Brazilian lignocellulosic wastes for bioenergy production: characterization and comparison with fossil fuels. BioResources 8(1):1166–1185
Yiga VA, Lubwama M, Olupot PW (2020) Investigation on char residues and mean reactivity of compression molded rice and coffee husks bio-char reinforced polypropylene. Begel House Inc, In ASTFE Digital Library
Lubwama M, Yiga VA (2018) Characteristics of briquettes developed from rice and coffee husks for domestic cooking applications in Uganda. Renew Energy 118:43–55
Lubwama M, Yiga VA (2017) Development of groundnut shells and bagasse briquettes as sustainable fuel sources for domestic cooking applications in Uganda. Renew Energy 111:532–542
Lima MDR, Simetti R, de Assis MR, Trugilho PF, Carneiro ADCO, Bufalino L, de Paula Protásio T (2020) Charcoal of logging wastes from sustainable forest management for industrial and domestic uses in the Brazilian Amazonia. Biomass and Bioenergy 142:105804
Lubwama M, Yiga VA, Lubwama HN (2020) Effects and interactions of the agricultural waste residues and binder type on physical properties and calorific values of carbonized briquettes. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-01001-8
Yiga VA, Pagel S, Lubwama M, Epple S, Olupot PW, Bonten C (2020) Development of fiber-reinforced polypropylene with NaOH pretreated rice and coffee husks as fillers: mechanical and thermal properties. J Thermoplast Compos Mater 33(9):1269–1291
Wilk M, Magdziarz A, Kalemba I, Gara P (2016) Carbonisation of wood residue into charcoal during low temperature process. Renew Energy 85:507–513
Pastor-Villegas J, Pastor-Valle JF, Rodriguez JMM, Garcia-Garcia M (2006) Study of commercial wood charcoals for the preparation of carbon adsorbents. J Anal Appl Pyrolysis 76:103–108
Júnior AFD, Suuchi MA, Neto ASA, da Silva JGM, da Silva ÁM, de Souza ND, Brito JO (2021) Blends of charcoal fines and wood improve the combustibility and quality of the solid biofuels. BioEnergy Res 14:344–354
Briseño-Uribe KC, Carrillo-Parra A, Bustamante-García V, González-Rodríguez H, Foroughbachk R (2015) Firewood production, yield and quality of charcoal from Eucalyptus camaldulensis and E. microtheca planted in the semiarid land of northeast Mexico. International Journal of Green Energy 12(9):961–969
Akowuah JO, Kemausuor F, Mitchual SJ (2012) Physico-chemical characteristics and market potential of sawdust charcoal briquette. Int J Energy Environ Eng 3:20. https://doi.org/10.1186/2251-6832-3-20
Ruiz-Aquino F, González-Peña MM, Valdez-Hernández JI, Revilla US, Romero-Manzanares A (2015) Chemical characterization and fuel properties of wood and bark of two oaks from Oaxaca, Mexico. Ind Crops Prod 65:90–95
European Committee for Standardization (2005) EN-1860–2: appliances, solid fuels and firelighters for barbecueing – Part 2: barbecue charcoal and barbecue charcoal briquettes. Requirements and test methods
Fernández RG, García CP, Lavín AG, de las Heras JLB (2012) Study of main combustion characteristics for biomass fuels used in boilers. Fuel Processing Technology 103:16–26
Chouchene A, Jeguirim M, Khiari B, Trouvé G, Zagrouba F (2010) Study on the emission mechanism during devolatilization/char oxidation and direct oxidation of olive solid waste in a fixed bed reactor. J Anal Appl Pyrolysis 87:168–174
Dorge S, Jeguirim M, Trouvé G (2011) Thermal degradation of Miscanthus pellets: kinetics and aerosols characterization. Waste Biomass Valor 2:149–155
Mitchual SJ, Frimpong-Mensah K, Darkwa NA (2014) Evaluation of fuel properties of six tropical hardwood timber species for briquettes. J Sustain Bioenergy Syst 4:1–9. https://doi.org/10.4236/jsbs.2014.41001
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261
Abreu-Naranjo R, Crespo YA, Pedretti EF, Conesa JA (2018) Experiments on torrefaction of Dichrostachys cinerea wood: two-level factorial design and thermogravimetric analysis. Wood Sci Technol 52:229–243
Kim N-H, Hanna RB (2006) Morphological characteristics of Quercus variabilis charcoal prepared at different temperatures. Wood Sci Technol 40:392–401
Pedrosa DT, Kaltschmitt M (2012) Dichrostachys cinerea as a possible energy crop—facts and figures. Biomass Convers Biorefin 2:41–51
Ijagbemi CO, Adepo SO, Ademol KS (2014) Evaluation of combustion characteristic of charcoal from different tropical wood species. IOSR J Eng 4(4):50–57
García R, Pizarro C, Lavín AG, Bueno JL (2014) Spanish biofuels heating value estimation Part II: proximate analysis data. Fuel 117:1139–1147
Jeguirim M, Elmay Y, Limousy L, Lajili M, Said R (2014) Devolatilization behavior and pyrolysis kinetics of potential Tunisian biomass fuels. Environ Prog Sustainable Energy 33(4):1452–1458
Das O, Sarmah AK, Bhattacharyya D (2016) Biocomposites from waste derived biochars: mechanical, thermal, chemical, and morphological properties. Waste Manage 49:560–570
Tribot A, Amer G, Alio MA, de Baynast H, Delattre C, Pons A, Dussap CG (2019) Wood-lignin: supply, extraction processes and use as bio-based material. Eur Polymer J 112:228–240
Idris SS, Abd Rahman N, Ismail K, Alias AB, Abd Rashid Z, Aris MJ (2010) Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Biores Technol 101(12):4584–4592
Lu KM, Lee WJ, Chen WH, Lin TC (2013) Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl Energy 105:57–65
Solar J, de Marco I, Caballero BM, Lopez-Urionabarrenechea A, Rodreguez N, Agirre I, Adrados A (2016) Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor. Biomass Bioenerg 95:416–423
Assis MR, Brancheriau L, Napoli A, Trugilho PF (2016) Factors affecting the mechanics of carbonized wood: literature review. Wood Sci Technol 50:519–536
Sadiku NA, Oluyege AO, Sadiku IB (2016) Analysis of the calorific and fuel value index of bamboo as a source of renewable biomass feedstock for energy generation in Nigeria. Lignocellulose 5(1):34–49
Limousy L, Jeguirim M, Labbe S, Balay F, Fossard E (2015) Performance and emissions characteristics of compressed spent coffee ground / wood chip logs in a residential stove. Energy Sustain Dev 28:52–59
Kwon S-M, Jang J-H, Lee S-H, Park S-B, Kim N-M (2013) Change of heating value, pH and FT-IR spectra of charcoal at different carbonization temperatures. J Korean Wood Sci Technol 41(5):440–446
Ding Z, Wan Y, Hu X, Wang S, Zimmerman AR, Gao B (2016) Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: importance of physicochemical properties. J Ind Eng Chem 37:261–267
Fu P, Hu S, Xiang J, Li P, Huang D, Jiang L, Zhang J (2010) FTIR study of pyrolysis products evolving from typical agricultural residues. J Anal Appl Pyrol 88(2):117–123
Magalhães D, Gürel K, Matsakas L, Christakopoulos P, Pisano I, Leahy JJ, Trubetskaya A (2021) Prediction of yields and composition of char from fast pyrolysis of commercial lignocellulosic materials, organosolv fractionated and torrefied olive stones. Fuel 289:119862
Usman AR, Abduljabbar A, Vithanage M, Ok YS, Ahmad M, Ahmad M, Al-Wabel MI (2015) Biochar production from date palm waste: charring temperature induced changes in composition and surface chemistry. J Anal Appl Pyrol 115:392–400
Waqas M, Aburiazaiza AS, Miandad R, Rehan M, Barakat MA, Nizami AS (2018) Development of biochar as fuel and catalyst in energy recovery technologies. J Clean Prod 188:477–488
Huang A, Zhou Q, Liu J, Fei B, Sun S (2008) Distinction of three wood species by Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. J Mol Struct 883:160–166
Pereira BLC, Oliveira AC, Carvalho AMML, Carneiro AO, Santos LC, Vital BR (2012) Quality of wood and charcoal from Eucalyptus clones for ironmaster use. Int J For Res. https://doi.org/10.1155/2012/523025
de Paula Protasio T, Lima MDR, Scatolino MV, Silva AB, Rodrigues de Figueiredo IC, Hein PRG, Trugilho PF (2021) Charcoal productivity and quality parameters for reliable classification of Eucalyptus clones from Brazilian energy forests. Renew Energy 164:34–45
Somerville M, Jahanshahi S (2015) The effect of temperature and compression during pyrolysis on the density of charcoal made from Australian eucalypt wood. Renew Energy 80:471–478
Desta HM, Ambaye CS (2020) Determination of energy properties of fuelwood from five selected tree species in tropical highlands of southeast Ethiopia. J Energy 2020:3635094. https://doi.org/10.1155/2020/3635094
Massuque J, De Assis MR, Trugilho PF (2020) Characterization of Miombo species used by rural communities as fuelwood in Northern Mozambique. Energy Sour Part A Recover Util Environ Eff 1–10. https://doi.org/10.1080/15567036.2020.1815910
Dufourny A, Van De Steene L, Humbert G, Guibal D, Martin L, Blin J (2019) Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. J Anal Appl Pyrol 137:1–13
Pérez-Peña N, Elustondo D, Valenzuela L, Ananías R (2020) Variation of perpendicular compressive strength properties related to anatomical structure and density in Eucalyptus nitens green specimens. BioResources 15(1):987–1000
Muhcu D, Terzi E, Kartal SN, Yoshimura T (2017) Biological performance, water absorption, and swelling of wood treated with nano-particles combined with the application of Paraloid B72®. Journal of forestry research 28(2):381–394
Acknowledgements
This work was funded by a grant from the African Institute for Mathematical Sciences, www.nexteinstein.org, with financial support from the Government of Canada, provided through Global Affairs Canada, www.international.gc.ca, and the International Development Research Centre, www.idrc.ca. Technical support from Yosevi Engineering Services Limited, www.yosevi.com, and the Materials and Metallurgy Laboratory at Busitema University Tororo, Uganda is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lubwama, M., Yiga, V.A., Ssempijja, I. et al. Thermal and mechanical characteristics of local firewood species and resulting charcoal produced by slow pyrolysis. Biomass Conv. Bioref. 13, 6689–6704 (2023). https://doi.org/10.1007/s13399-021-01840-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01840-z