Abstract
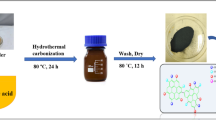
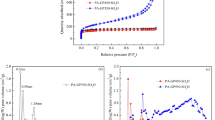
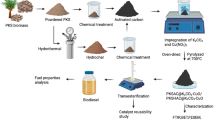
The current study describes the preparation and catalytic activity evaluation of newly synthesized hydrothermal carbon catalysts for biodiesel production. The catalysts (HTCC-M2-10h) were prepared through the sulfonation of mango peels (Mangifera indica L.) using concentrated sulfuric acid at various time intervals from 2- to 10-h process. The catalysts were characterized via sophisticated analytical techniques to analyze surface area, functional groups, morphology, and acid density sites. The catalyst prepared at a 6-h time interval shows a high density of proton switchable/acidic sites attached to the carbon surface (i.e., SO3H, COOH, and OH). The catalyst was found to exhibit a large surface area (42.13 m2/g), well-ordered porosity, and large pore volume. The catalytic activity was determined by examining the catalyst performance for fatty acid esterification to produce the methyl ester (i.e., biodiesel). Thus, HTCC-M6h (prepared at 6 h) gives the maximum catalytic activity due to its high acid density (3.46 mmol/g) and large surface area. The maximum 98.6% oleic acid conversion into methyl oleate was obtained at 65 °C by using 10:1 v/v methanol:oil and 5 wt% of catalyst within 4 h. The kinetic study for oleic acid esterification by HTCC-M6h performed at temperatures between 40 and 70 °C confirmed the first-order reaction as is the case for homogeneous esterification reaction. The recyclable ability of catalyst was also determined and found that the catalyst has enough potential to recycle up to four times without any reactivation step and any activity loss after that catalyst can be regenerated. The results revealed that the HTCC-M6h exhibits high stability, high catalytic activity, and reusability. It could be concluded that the prepared HTCC-M6h will prove itself an innovation in heterogeneous catalysis.






Similar content being viewed by others
References
Lee AF, Bennett JA, Manayil JC, Wilson K (2014) Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem Soc Rev 43(22):7887–7916
Su F, Guo Y (2014) Advancements in solid acid catalysts for biodiesel production. Green Chem 16(6):2934–2957
Birla A, Singh B, Upadhyay S, Sharma Y (2012) Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour Technol 106:95–100
Abelniece Z, Laipniece L, Kampars V (2020) Biodiesel production by interesterification of rapeseed oil with methyl formate in presence of potassium alkoxides. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00874-z
Park J-Y, Kim D-K, Lee J-S (2010) Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour Technol 101(1):S62–S65
Corro G, Tellez N, Ayala E, Marinez-Ayala A (2010) Two-step biodiesel production from Jatropha curcas crude oil using SiO2· HF solid catalyst for FFA esterification step. Fuel 89(10):2815–2821
Yan S, Salley SO, Ng KS (2009) Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl Catal A Gen 353(2):203–212
Chen G, Fang B (2011) Preparation of solid acid catalyst from glucose–starch mixture for biodiesel production. Bioresour Technol 102(3):2635–2640
Guo F, Fang Z, Tian X-F, Long Y-D, Jiang L-Q (2011) One-step production of biodiesel from high-acid value Jatropha oil in ionic liquids.
Arata K (2009) Organic syntheses catalyzed by superacidic metal oxides: sulfated zirconia and related compounds. Green Chem 11(11):1719–1728
Reddy BM, Patil MK (2009) Organic syntheses and transformations catalyzed by sulfated zirconia. Chem Rev 109(6):2185–2208
Melero JA, Iglesias J, Morales G (2009) Heterogeneous acid catalysts for biodiesel production: current status and future challenges. Green Chem 11(9):1285–1308
Wu M, Wang Y, Wang D, Tan M, Li P, Wu W, Tsubaki N (2016) SO 3 H-modified petroleum coke derived porous carbon as an efficient solid acid catalyst for esterification of oleic acid. J Porous Mater 23(1):263–271
Andrijanto E, Dawson E, Brown D (2012) Hypercrosslinked polystyrene sulphonic acid catalysts for the esterification of free fatty acids in biodiesel synthesis. Appl Catal B Environ 115:261–268
Zuo D, Lane J, Culy D, Schultz M, Pullar A, Waxman M (2013) Sulfonic acid functionalized mesoporous SBA-15 catalysts for biodiesel production. Appl Catal B Environ 129:342–350
Pirez C, Lee A, Manayil J, Parlett C, Wilson K (2014) Hydrothermal saline promoted grafting: a route to sulfonic acid SBA-15 silica with ultra-high acid site loading for biodiesel synthesis. Green Chem 16(10):4506–4509
Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica-based mesoporous organic–inorganic hybrid materials. Angew Chem Int Ed 45(20):3216–3251
Kulkarni MG, Gopinath R, Meher LC, Dalai AK (2006) Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem 8(12):1056–1062
Srilatha K, Issariyakul T, Lingaiah N, Sai Prasad P, Kozinski J, Dalai A (2010) Efficient esterification and transesterification of used cooking oil using 12-tungstophosphoric acid (TPA)/Nb2O5 catalyst. Energy Fuel 24(9):4748–4755
Hara M, Yoshida T, Takagaki A, Takata T, Kondo JN, Hayashi S, Domen K (2004) A carbon material as a strong protonic acid. Angew Chem Int Ed 43(22):2955–2958
Suganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, Hayashi S, Hara M (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc 130(38):12787–12793
Okamura M, Takagaki A, Toda M, Kondo JN, Domen K, Tatsumi T, Hara M, Hayashi S (2006) Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem Mater 18(13):3039–3045
Toda M, Takagaki A, Okamura M, Kondo JN, Hayashi S, Domen K, Hara M (2005) Green chemistry: biodiesel made with sugar catalyst. Nature 438(7065):178
Su DS, Perathoner S, Centi G (2013) Nanocarbons for the development of advanced catalysts. Chem Rev 113(8):5782–5816
Titirici M-M, White RJ, Brun N, Budarin VL, Su DS, del Monte F, Clark JH, MacLachlan MJ (2015) Sustainable carbon materials. Chem Soc Rev 44(1):250–290
Lam E, Luong JH (2014) Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal 4(10):3393–3410
Chang B, Tian Y, Shi W, Liu J, Xi F, Dong X (2013) SO 3 H-functionalized mesoporous carbon/silica composite with a spherical morphology and its excellent catalytic performance for biodiesel production. J Porous Mater 20(6):1423–1431
Chang B, Li Y, Guo Y, Yin H, Zhang S, Yang B (2015) SO 3 H-functionalized hollow mesoporous carbon sphere prepared by simultaneously achieving sulfonation and hollow structure. J Porous Mater 22(3):629–634
Hu B, Xiong C, Tao K, Zhou S (2015) Metathesis of 1-butene and ethene to propene over mesoporous W-KIT-6 catalysts: the influence of Si/W ratio. J Porous Mater 22(3):613–620
Liu F, Sun J, Zhu L, Meng X, Qi C, Xiao F-S (2012) Sulfated graphene as an efficient solid catalyst for acid-catalyzed liquid reactions. J Mater Chem 22(12):5495–5502
Oliveira BL, da Silva VT (2014) Sulfonated carbon nanotubes as catalysts for the conversion of levulinic acid into ethyl levulinate. Catal Today 234:257–263
Gao Z, Tang S, Cui X, Tian S, Zhang M (2015) Efficient mesoporous carbon-based solid catalyst for the esterification of oleic acid. Fuel 140:669–676
FAOSTAT F statistics, food and agriculture organization of the United Nations, Rome, Italy, 2004.
Silva APM, Oliveira AV, Pontes SM, Pereira AL, Rosa MF, Azeredo HM (2019) Mango kernel starch films as affected by starch nanocrystals and cellulose nanocrystals. Carbohydr Polym 211:209–216
Joseph J, Abolaji J (1997) Effects of replacing maize with graded levels of cooked Nigerian mango-seed kernels (Mangifera indica) on the performance, carcass yield and meat quality of broiler chickens. Bioresour Technol 61(1):99–102
Laskar IB, Gupta R, Chatterjee S, Vanlalveni C, Rokhum L (2020) Taming waste: waste Mangifera indica peel as a sustainable catalyst for biodiesel production at room temperature. Renew Energy 161:207–220. https://doi.org/10.1016/j.renene.2020.07.061
Gohain M, Laskar K, Phukon H, Bora U, Kalita D, Deka D (2020) Towards sustainable biodiesel and chemical production: multifunctional use of heterogeneous catalyst from littered Tectona grandis leaves. Waste Manag 102:212–221
Román S, Nabais J, Laginhas C, Ledesma B, González J (2012) Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process Technol 103:78–83
Broch A, Jena U, Hoekman SK, Langford J (2014) Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 7(1):62–79
Tang X, Niu S (2019) Preparation of carbon-based solid acid with large surface area to catalyze esterification for biodiesel production. J Ind Eng Chem 69:187–195
Rocha PD, Oliveira LS, Franca AS (2019) Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renew Energy 143:1710–1716
Tamborini LH, Casco ME, Militello MP, Silvestre-Albero J, Barbero CA, Acevedo DF (2016) Sulfonated porous carbon catalysts for biodiesel production: clear effect of the carbon particle size on the catalyst synthesis and properties. Fuel Process Technol 149:209–217
Xing R, Liu Y, Wang Y, Chen L, Wu H, Jiang Y, He M, Wu P (2007) Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials. Microporous Mesoporous Mater 105(1-2):41–48
Tsubouchi N, Xu C, Ohtsuka Y (2003) Carbon crystallization during high-temperature pyrolysis of coals and the enhancement by calcium. Energy Fuel 17(5):1119–1125
Lou W-Y, Zong M-H, Duan Z-Q (2008) Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour Technol 99(18):8752–8758
Liu X-Y, Huang M, Ma H-L, Zhang Z-Q, Gao J-M, Zhu Y-L, Han X-J, Guo X-Y (2010) Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 15(10):7188–7196
Kruk M, Jaroniec M, Sayari A (1997) Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 13(23):6267–6273
Nguyen HC, Ong HC, Pham TTT, Dinh TKK, Su CH (2020) Microwave-mediated noncatalytic synthesis of ethyl levulinate: a green process for fuel additive production. Int J Energy Res 44(3):1698–1708
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86(10):1059–1070
Mo X, Lotero E, Lu C, Liu Y, Goodwin JG (2008) A novel sulfonated carbon composite solid acid catalyst for biodiesel synthesis. Catal Lett 123(1-2):1–6
Yu H, Niu S, Lu C, Li J, Yang Y (2016) Preparation and esterification performance of sulfonated coal-based heterogeneous acid catalyst for methyl oleate production. Energy Convers Manag 126:488–496
Lou WY, Guo Q, Chen WJ, Zong MH, Wu H, Smith TJ (2012) A highly active bagasse-derived solid acid catalyst with properties suitable for production of biodiesel. ChemSusChem 5(8):1533–1541
Hood ZD, Adhikari SP, Li Y, Naskar AK, Figueroa-Cosme L, Xia Y, Chi M, Wright MW, Lachgar A, Paranthaman MP (2017) Novel acid catalysts from waste-tire-derived carbon: application in waste-to-biofuel conversion. Chem Select 2(18)
Tumkot L, Quitain AT, Kida T, Laosiripojana N, Boonnoun P (2020) Sulfonated hydrothermal carbon-based catalyzed esterification under microwave irradiation: optimization and kinetic study. Bull Chem React Eng Catal 15(2):514–524
Liu W, Yin P, Liu X, Chen W, Chen H, Liu C, Qu R, Xu Q (2013) Microwave assisted esterification of free fatty acid over a heterogeneous catalyst for biodiesel production. Energy Convers Manag 76:1009–1014
Lieu T, Yusup S, Moniruzzaman M (2016) Kinetic study on microwave-assisted esterification of free fatty acids derived from Ceiba pentandra Seed Oil. Bioresour Technol 211:248–256
Zhang H, Ding J, Qiu Y, Zhao Z (2012) Kinetics of esterification of acidified oil with different alcohols by a cation ion-exchange resin/polyethersulfone hybrid catalytic membrane. Bioresour Technol 112:28–33
Abbas AS, Abbas SM (2013) Kinetic study and simulation of oleic acid esterification in different type of reactors. Iraqi J Chem Petrol Eng 14(2):13–20
Funding
This work was supported and funded from the Project Pas-US project (No: 6-6/Pak-US/HEC/2015/6) granted under Pak-US Science and Technology Program and National Centre of Excellence in Analytical Chemistry (NCEAC), University of Sindh Jamshoro, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 38 kb)
Rights and permissions
About this article
Cite this article
Memon, S.S., Memon, N., Memon, S. et al. An excellent sulfonated hydrothermal carbon catalyst from Mangifera indica L. (mango peels) for biodiesel production: preparation, characterization, optimization, and kinetic study. Biomass Conv. Bioref. 12, 141–151 (2022). https://doi.org/10.1007/s13399-021-01535-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01535-5