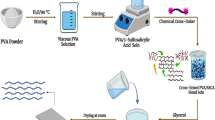
Abstract
This work presents the preparation and investigation of blended nylon (N)/polyvinyl alcohol (PVA)-based polyelectrolytic membranes that are modified with different concentrations of sulfuric acid (SA), chlorosulfonic acid (CSA), and sulfonated activated carbon (SAC) as a filler. Scanning electron microscopy (SEM) micrographs illustrated good membrane homogeneity, and no cracks or phase separation were detected. Chemical interaction between N, PVA, and other membrane components was confirmed by Raman scattering spectroscopy and Fourier transform infrared (FTIR). In addition, the molecular structure is verified by energy depressive X-ray (EDX). Furthermore, water and methanol uptake, gel fraction, and IEC were determined as functions of varied membrane modification components. The results revealed that increasing the portion of SA, CSA and SAC led to an increase in IEC and ionic conductivity values reached 2.12 meq/g–0.076 S/cm for (N/PVA-4.0% SA-4.0% SAC), respectively, and 2.71 meq/g–0.087 S/cm for (N/PVA-4.0% CSA-4.0% SAC), respectively, while the IEC and ionic conductivity value for non-modified N/PVA membrane was 0.02 meq/g and zero, respectively. Such results enhance the potential feasibility of modified N/PVA electrolytic membranes for fuel cell (FC) applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the important promising technologies for clean and efficient power sources in the twenty-first century is the proton exchange membrane fuel cells (PEMFCs). Their importance is attributed to the capability of providing electricity for a wide range of applications such as automotive transportation, military, and portable electronics. The widespread commercialization and uses limitation of these alternative power sources due to fueling problems, lifetime, and installation cost [1, 2].
PEMFC is an electrochemical cell that uses hydrogen as fuel or hydrogen in rich materials such as methanol which is oxidized at the anode to produce electrons and protons (H+) in general. Protons are conducted to the cathode through a polymeric electrolyte which is an electrical insulator. On the other hand, oxygen is reduced at the cathode and combined with H+ to produce water. The electrons passed in outer circuits to the other providing electric current [3]. Due to significant efficiency, lightness, and fuel viability, direct methanol fuel cells (DMFCs) have extreme potential in generating electricity. However, some technical limitations restrict the commercialization and dispreading of the DMFC [4], such as the high methanol crossover through polymeric electrolyte membrane and the slow oxidation kinetics of methanol in addition to the high cost of membrane production [5].
Owing to the good thermal stability and good conductivity of perfluorosulfonic acid (PFSA) group membranes, such as Nafion have been commercially used in PEMFC applications as an electrolyte membrane [6]. However, the high production cost of Nafion limits its industrial applications [4]. The low-proton conductivity at high temperatures, fast dehydration [7], and high methanol crossover even at room temperature is the key problem for the usage of Nafion in DMFC [8].
PVA and polyamides such as nylon 6,6 polymers are supported to use in wide applications such as selective membranes and ion exchange membranes [9] due to these polymers show good film-forming properties with chemical and mechanical stability and high-density hydrophilic groups allowing chemical modifications of the polymer chain by crosslinking, grafting or sulfonation [10] rather than its non-toxicity and cost-effectiveness [11]. The uses of PVA expose some advantages such as ease of preparation, biocompatibility, good flexibility, high hydrophilic, and high abrasion resistance [12]. Furthermore, PVA displayed very low ion exchange capacity (IEC) rather than ionic conductivity compared to Nafion owing to the absence of any negatively charged ions, like sulfonic (-SO3H) or carboxylic acid (-COOH) groups in their structure. Therefore, PVA membranes can be utilized in a fuel cell if the groups that hold negative ions are embedded in their structure to increase their conductivity [13, 14]. Regarding methanol crossover, the addition of fillers such as SiO2 [15], polyrotaxane [16], montmorillonite [17], and graphene oxide (GO) [18] into the polymer matrix contributes to mitigating this issue.
Sulfonated PVA prepared from 4-formylbenzene- 1,3-disulfonic acid and crosslinked with 4,4-oxydiphthalic anhydride and1,3-bis(3-glycidyloxypropyl) tetramethyldisiloxane. These membranes presented good proton conductivity (0.218 S cm−1 at 70 °C) and low methanol permeability (at around 1.25 × 10–6 cm2 s−1), then they can be served in DMFC [19].
A series of sulfonated polyimides (SPIs) were prepared using a different sulfonating agent as 4,4-diaminobiphenyl-2,2-disulfonic acid and 2,5-diamino benzene sulfonic acid [20]. The prepared membranes exhibit IEC values of about 1.6 mmol/g and appeared lower conductivities than Nafion® 1135 at 80 ◦C and all relative humidity (RH) levels [21]. In DMFC measurements the membranes revealed similar performance as Nafion® 117 at 80 ◦C with the methanol feed concentration of 0.5 M [21, 22].
One of the ways that are used to improve ionic conductivity, mechanical and thermal stability is the introducing of organic or inorganic materials into the polymer matrix to prepare a composite membrane. One of the attractive materials that have a great potential to produce composite membranes is the activated carbon (AC) molecular sheet [18, 23, 24]. The advantage of AC is attributed to its excellent thermal [25], mechanical [26], transport [27], and gas barrier [28]. However, AC is difficult to exfoliate in a polymer matrix due to its high cohesive force between them. Thus, AC is functionalized with other groups such as (-SO3H) to impart specific features and interactions [29].
Therefore, the research is conducted to find an alternative membrane for the proton exchange membrane for fuel cells based on sulfonated polyimides and PVA in this work. We show that the blending of a polyamide such as nylon 6,6 with PVA followed by sulfonation using SA or CSA and sulfonated activated carbon (SAC) addition strategy are important for blocking methanol crossover and improvement of IEC and therefore, ionic transportation property of the membrane with enhanced electrochemical performance.
2 Materials and Methods
2.1 Materials
Nylon 6,6 (MW ≥ 226.14 g/mol, density = 1.47 g/ml at 25 °C, melting point 250–260 °C) was obtained from Merck, Germany. PVA (typically average MW = 124,000 g/mol, 95–96.5% hydrolyzed) was supplied from Fisher Scientific, UK. Chlorosulfonic acid (MW = 116.52 g/mol, purity 99%, density 1.753 g/mL at 25 °C) was received from Merck, Germany. Formic acid (M = 46.03 g/mol, purity 98–100%, density 1.22 g/ml) was bought from BDH chemicals Ltd, England. Activated carbon (Mw = 12.01 g/mol, sulfur content 0.15%, acid solubility 2.0%) was purchased from Uni-CHEM chemical reagents, South Korea.
2.2 Preparation of N/PVA-Based Polyelectrolytic Membranes
10 wt% of Nylon 6,6 solution was prepared by dissolving the pre-weighed amount of Nylon 6,6 in formic acid at room temperature with stirring for 2 h till the solution becomes homogenous. On the other hand, 10 wt% of PVA solution was prepared in a similar way that was mentioned for Nylon 6,6. Both solutions of Nylon 6,6 and PVA were stored at room temperature. The suggested reaction that takes place between Nylon 6,6 and PVA is shown in Scheme 1.
Sulfonated activated carbon (SAC) was prepared according to the method reported by Bermejo et al. [30] by mixing 10 g of pure activated carbon with 100 mL of sulfuric acid, under stirring at 160 °C for 8 h. Then, the mixture was washed several times with deionized water and allowed to dry at 60 °C for 6 h. Finally, SAC was packed and stored at room temperate for the next use.
The membrane was prepared by mixing 5 mL of Nylon 6,6 with the same amount of PVA solution. After that, the mixture was vigorously stirred at 40 °C for 4 h. The previous steps were repeated to prepare 15 samples for use in the next steps. First, the various amounts (v/v %) of sulfuric acid (SA) and chlorosulfonic acid (CSA) (1%, 2%, 3%, and 4%) each of them stepped wise added to four samples during the stirring process, and the temperature was raised to 65 °C for 4 h. Second, 4% of SA and CSA was added slowly to three samples under stirring condition and temperature within 65 °C for 4 h, then various amounts (w/v %) of SAC (1%, 2%, and 4%) was added under the previous condition for extra 2 h. The remain sample is still without any additives. All mixtures were cast onto a polypropylene sheet. The solvent evaporated at room temperature for 12 h. Then, the casted membranes were allowed to dry at 70 °C for 6 h. Finally, the dried membranes were subjected to different characterization methods.
2.3 Characterization
2.3.1 Spectral Analysis
The chemical bonding and functional groups within the N/PVA and modified N/PVA-based polyelectrolytic membranes were evaluated using (Shimadzu FTIR-8400 S, Japan) with a resolution of 4 cm−1 and a wavenumber range of 400–4000 cm−1 [31]. Additionally, a laser Raman scattering spectrometer (SENTERRA-Bruker, Germany) equipped with a Leica microscope was also used to investigate the chemical bonding and possible interactions inside the prepared polymeric membranes [32].
2.3.2 Scanning Electron Microscopic (SEM)
Morphological features and microstructure of modified N/PVA-based polyelectrolytic membranes were investigated using SEM, (JEOL JSM-6360LA, Japan). SEM was in operation at an acceleration voltage of 15 kV. Magnification power varied from 500 to 5000 [33, 34].
The elemental composition analysis of modified N/PVA-based polyelectrolytic membranes was determined using an energy dispersive X-ray spectrometer (EDX) attached to the scanning electron microscope [35].
2.3.3 Mechanical Properties
Tensile strength and the elongation at break of modified N/PVA-based polyelectrolytic membranes have been conducted at ambient temperature using a universal Testing Machine (Shimadzu UTM, Japan). Tensile and elongation measurements were carried out with crosshead movement at a constant speed of 3 mm/min for specimens of 30 × 10 mm [34].
2.3.4 Water and Methanol Uptake
Water and methanol uptake of modified N/PVA-based polyelectrolytic membranes are usually defined in weight percent with respect to the weight of the dried membrane. To determine the swelling ability of N/PVA and modified N/PVA-based polyelectrolytic membranes, specimens of these membranes were obtained by cutting specified membrane samples into 3.0 × 3.0 cm pieces. The specimens were dried for 6 h at 90 °C. After that, it was weighted (Wdry). Then the dried samples were soaked in distilled water or methanol for 12 h at room temperature to determine the water and methanol uptake ratio, respectively. Afterward, swelled samples were leached from those liquids and weighted again (Wwet). The swelling ratio is determined by Eq. (1) [34, 36].
where (Wdry) and (Wwet) are the weights of dried membrane samples before and after soaking, respectively, in desired liquid.
2.3.5 Determination of Gel Fraction
The modified N/PVA-based polyelectrolytic membranes are dried at room temperature to avoid any surface shrinking for 12 h and weighed (W1), then immersed in distilled water for another 24 h, up to an equilibrium swelling weight, to remove the leachable or soluble components. The polyelectrolytic membranes are then dried and weighed again (W2). The gel fraction percent was carried out according to the method reported by Fahmy et al. [37] and calculated by Eq. (2).
where (W1) and (W2) are the weights of dried membrane samples before and after soaking, respectively.
2.3.6 Determination of Ion Exchange Capacity
Ion exchange capacity (IEC) represents the total of active sites or functional groups responsible for ion exchange in N/PVA and modified N/PVA-based polyelectrolytic membranes. In most cases, the IEC is determined using a standard acid–base titration technique. Weighed samples were immersed in 20 cm3 of a 2 M (NaCl) solution for 12 h at 25 °C. The solution was then titrated with a known concentration of NaOH [38]. IEC (in Meq/g) is determined by using Eq. (3):
where N, V, and W are the concentration of the NaOH solution, the titer of the NaOH solution, and the weight of the sample, respectively.
2.3.7 Determination of Ionic Conductivity
Proton conductivity of modified N/PVA-based polyelectrolytic membranes was performed using impedance spectroscopy using a Solartron 1260 gain phase analyzer, interfaced to a Solartron 1480 multistate. The protonic conductivity (σ) of the membranes is calculated using Eq. (4) from impedance data [39].
where L is the distance between electrodes, R is the membrane resistance, and D and W are the thickness and width of the membranes, respectively.
2.3.8 Determination of Electrical Conductivity
Electrical conductivity of the prepared membranes was performed according to the method reported by Bazli et al. [40] with modification. The membrane specimen with a thickness of ca. 0.8 mm was immersed in a mixture of deionized water and methanol in a (1:1) ratio for 2 h at ambient temperature. Then, the membrane specimen is placed between two flattened copper electrodes and an incremental alternating voltage of (5, 10, 20 and 40 V) was applied. Finally, the tested specimen was notified to withstand the flow of current.
3 Results and Discussion
SA and CSA are used in the separate systems as modifying agents to incorporate sulfonic (-SO3H) groups into carbon, nitrogen, or oxygen atom of N/PVA chains through sulfonation reaction [41]. The presence of the -SO3H group in the N-PVA polymer matrix can be improving hydrophilicity, IEC and ionic conductivity.
The role of SAC addition is not only to achieve materials with better mechanical properties but to enhance a composite material with good barrier properties for penetration of fuel [23] in DMFC. In general, the mixing process of clay with polymer hydrogel matrix yields a composite hydrogel with could bear more external load than the pure hydrogel rather than good barrier properties for penetration of fuel [24] as shown in Scheme 2. However, pristine AC contains no surface functional group and has very limited dispersibility in the nylon matrix, seriously restricting its potential application in the preparation of functional composites. Therefore, chemical modification is needed for AC to achieve a homogeneous dispersion in the nylon matrix and optimize ionic conductivity [43]. An early experimental result from Hara’s group [44] showed that heating AC in H2SO4 produced a carbon material with a -SO3H group density of about 0.15 mmol·g−1 [45]. Onda et al. reported the generation of sulfonated carbon material with a -SO3H density of 0.44 mmol·g−1 by treatment of AC with concentrated H2SO4 [46]. The sulfonated activated carbon SAC obtained, in this case, was quite stable and showed evident catalytic activity [45]. Undoubtedly, it is advantageous to prepare N/PVA-SAC composites with good dispersibility and excellent properties.
Illustrate the pathway of material through the composite membrane, a in the presence of SAC, and b in the absence of SAC [42]
3.1 FTIR Spectra of N/PVA and Modified N/PVA-Based Polyelectrolytic Membranes
FTIR spectra of N-PVA membranes within the range of 500–4000 cm−1 wavenumber are shown in Fig. 1. FTIR spectrum of pristine N-PVA polyelectrolytic membrane is shown in Fig. 1a. In this spectrum, a beak around 3300–3500 cm−1 was observed which was attributed to the stretching vibration of the OH groups within the structure of PVA, where a weak peak that was detected around 1400 cm−1 was related to O–H deformation; thus, it might be related to bond formation [47].
The weak broad peak around 2900 cm−1 is related to the symmetric and asymmetric CH of the polymer backbone [48]. In addition, the peak which was recorded in the range of 1500–1700 cm−1 is an indication of the existence of the C=O group of the amide bond in Nylon 6,6 and the O–C–O bond formed between N and PVA [36, 49, 50] shown in Scheme. 1. Meanwhile, the spectrum of sulfonated N-PVA membranes revealed two peaks (b) at 1139 cm−1 for SA sulfonating agent and (c) at 1141 cm−1 for CSA sulfonating agent due to the S=O stretching of sulfonic groups. Strong two peaks at around 1070 cm−1 and 1065 cm−1 appeared on (d) and (e), respectively, due to the S=O stretching of sulfonic groups [51] in the SAC or O–S=O in the -SO3H group [52]. The band around 1190 cm−1 is related to the S–O bond. It was noticed that the intensity of the S=O peak increased as the proportion of sulfonating agents increased [53]. As stated above, the results obtained from FTIR confirmed the presence of various functional groups such as –OH, –COOH, –COO–, –SO3H, and –CO, within the polymer blended structure.
Additionally, the side reactions of formic acid (solvent) with amine groups in nylon 6,6 in the presence of sulfuric acid may be eliminated CO2 molecules that appeared in FTIR spectra near 2400 cm−1, and the intensity of this peak is increased with increasing sulfuric acid molar ratio [54, 55].
3.2 Raman Spectra
Raman scattering spectra of modified N/PVA-based polyelectrolytic membranes are shown in Fig. 2. The spectrum of SA- or CSA-based membrane revealed a strong valence band at 1053 cm−1 due to the stretching SO3 group, and the intensity of this peak was increased as a portion of the acid in the polymer blend was increased. This increase is attributed to an increase in the -SO3H group in the N-PVA-based membranes [56].
Raman spectra of modified N-PVA-based polyelectrolytic membranes: N/PVA-(1%) SA (A), N/PVA-(2%)SA (B), N/PVA-(3%) SA (C), N/PVA-(4%) SA (D); b N/PVA-(1%) CSA (E), N/PVA-(2%)CSA (F), N/PVA-(3%) CSA (G), N/PVA-(4%) CSA (H);c N/PVA-(4%) SA (I), N/PVA-(4%)SA- (1%)SAC (J), N/PVA-(4%)SA-(2%)SAC (K), N/PVA-(4%)SA-(4%)SAC (L);d N/PVA-(4%) SA (M), N/PVA-(4%)CSA- (1%)SAC (N), N/PVA-(4%)CSA-(2%)SAC (O), N/PVA-(4%)CSA-(4%) SAC (P)
Other signals that appeared at 1106 cm−1 and 1445 cm−1 correspond to the vibration of C–C and C-H bonds, respectively, whereas Raman spectra of N-PVA-based membranes exhibited three characteristic scattering peaks at 1423, 1208, and 653 cm−1 which were belonging to a stretching N–H bond [31]. The spectrum showed a strong valence band at 1662 cm−1 due to the C=O bond in the main chain of Nylon [34, 57].
3.3 SEM Micrographs
The morphology of the surface and cross section of modified N/PVA-based polyelectrolytic membranes was examined using SEM compared to pure N/PVA as a blank. Figure 3A shows typical SEM images of pristine N/PVA in comparison with modified N/PVA membrane using SA (Fig. 3B) and CSA (Fig. 3C). The sulfonated N/PVA-based membranes revealed generally a quite homogenous surface compared to non-modified N/PVA due to the sulfonic group insertion causing tying and alignment of polymer chains. Cross-sectional SEM micrographs of N/PVA and its sulfonated form-based membranes micrographs exhibited clearly that the microstructure of the cross section of the N/PVA-based membrane was wax-like (Fig. 3A cross section); this property turned to spongy-like with the sulfonated N/PVA membrane. The observed changes in the morphological surface and cross section are an indication for the sulfonation process which greatly affects the membrane structure and properties. However, the microstructure of all sulfonated N/PVA-based membranes was denser compared with the pristine N/PVA-based membrane. These findings can be originated from the hydrogen bonding between polymer chains bearing hydrophilic groups [31, 50]. On the other hand, morphological features of N/PVA-SA and N/PVA-CSA membranes that blended with SAC are shown in Fig. 3D, E, respectively. These micrographs elucidated that these membranes have a homogeneous and compact structure with a rougher surface than pristine N/PVA membrane and no phase separation where its surface was uniform and free from any cracks. These characteristics might be related to good compatibility and strong interfacial interactions between N/PVA and SAC platelets [47]. It is worth mentioning here that surface pores also appeared on the surface of micrographs and not extended across the membrane, and this may be leading to the smooth transfer of protons through the membrane [49] and preventing methanol crossover.
3.4 EDX Analysis
EDX microanalysis is a technique used for the identification of the elemental composition of specimens. EDX is capable of generating a map of one or more chemical elements of interest [52]. Analysis of N/PVA-based polyelectrolytic membranes with an EDX spectrometer confirmed the presence of elemental C, N, O, and S. The carbon, oxygen, and nitrogen in the examined samples are attributed to the matrix of PVA and nylon as well as the addition of activated carbon. Nitrogen does not appear in EDX analysis due to its minor existence. As given in Fig. 4a, carbon and oxygen are the main content of the blank sample. The sulfonated N/PVA using the SA agent exhibited increase in the S element band with an increase in SA molar ratio (Fig. 4b, c, d) indicating that the sulfonation process occurred successfully [53]. The behavior is in harmony with the increase in the SAC molar ratio. These observations confirmed that SAC is not affected by the preparation process as well as good compatibility between SAC and holder N/PVA matrix as explained in the previous section (SEM). On the other hand, the amount of S elemental increases to a higher percentage at the maximum loading of SAC (4%) in the N/PVA-SA polyelectrolytic membrane (Supplementary 1). This behavior demonstrates the success of the treatment process of activated carbon with sulfonating agent [58]. Supplementary 2 exhibits the comparison between two concentrations of SAC (1 and 2%) in the N/PVA-CSA electrolytic membrane. As shown, the amount of elemental S increased with an increase in the molar ratio of SAC which, in turn, could be taken to indicate the success of the insertion of SAC into the holder matrix and increasing the number of polar groups such as -SO3H.
3.5 Elemental Analysis
Carbon, hydrogen, nitrogen, and sulfur content in the modified N/PVA electrolytic membranes was evaluated by (CHNS) analyzer. Results obtained from modified N/PVA-based polyelectrolytic membranes with different ratios of SA are presented in Table 1. The N/PVA membrane is rich in carbon, hydrogen, nitrogen, and traces of sulfur elements suggesting a small number of functional groups present in the membrane that is attached to the main backbone of nylon and PVA.
Sulfonation of N/PVA membrane with different amounts of SA results in a slight loss of carbon and enrichment of the samples in sulfur with an increase in SA content nearly of the same percent. This behavior conducts to the success of the preparation method for the sulfonation process of N/PVA membranes, and there are no side reactions that affect the polymer matrix causing degradation or fragmentations which are also confirmed by C/N and C/H ratios [59, 60]. Consequently, the result of the modified N/PVA membrane using CSA is represented in Table 1. The results are in a significant similar behavior of SA; the amount of sulfur element increased with the addition of CSA indicating more sulfonic group attached to the N/PVA polymeric matrix.
The effect of SAC addition to N/PVA-4% SA and N/PVA-4% CSA polyelectrolytic membranes, respectively, is presented in Table 1. As result, C/N and C/H ratios translate the composition stability of membranes after the addition of SAC, and there is no notified loss in the carbon of the backbone. On the other hand, the C/S ratio decreased with the increase in SAC content indicating a more polar group (–SO3H) which shifts membranes to more ion exchange capacity and exponential surface area [60]. These scenarios were founded in more value in the case of sulfonation using CSA at maximum loading of SAC.
3.6 Tensile Strength Measurements
The mechanical properties of N/PVA and modified N/PVA electrolytic membranes are shown in Table 2. These findings indicated that the tensile strength and elongation at break of the pristine N/PVA membrane were found in low values equal to 26.3 MPa and 178.4%, respectively. These results are compatible with others obtained in gel fraction in Fig. 6a which declared that the N/PVA membrane has lower crosslinking density due to no further modification or crosslinking of two polymeric matrices and the van der Walls forces worked probably. Modified N/PVA polyelectrolytic membrane was noticeably decreased in tensile strength with the sulfonation process. Furthermore, this decline was more pronounced with an increase in the degree of sulfonation resulting from an increase in the molar concentration of SA or SAC used in this process. Furthermore, the blending of SAC with sulfonated N/PVA membranes led to an increase in tensile strength to a certain extent and then decreases again for those membranes. This reduction was increased with increasing the proportion of SAC in sulfonated N/PVA-based membranes. On the contrary, elongation at break exhibits increase with decreasing tensile strength in the sulfonated membrane using SA or CSA and exposes similar behavior in line with a tensile strength with an increase in SAC portion in sulfonated N/PVA electrolytic membranes. The above founding can be explained on the basis that the potential interactions between the blended components (N and PVA) were lower in the case of pristine N/PVA membranes compared with that in N/PVA-SA-, N/PVA-SA-SAC-, N/PVA-CSA- or N/PVA-CSA-SAC-based electrolytic membranes. The addition of SA or CSA improves the interaction between two polymer components as shown in Scheme 1a which presents the chemical interaction between N and PVA to form ether bond -O- which is confirmed by FTIR. Scheme 2 also explains the ionic crosslinking between N/PVA in the presence of SA or CSA [31, 61]. The addition of SAC filler led to composing of many SAC sheets held together by van der Waals forces to form rigid platelets several hundred thick making it unable to interact well with polymeric matrix [62] leading to deterioration in mechanical properties.
3.7 Water Uptake
Ionic or proton conductivity is highly dependent on the water content retained by the membrane, where the hydration of PEM is an influential factor to enhance their proton conductivity and subsequently improve the performance of the fuel cell [31]. Figure 5a displays the total water content in N/PVA and modified N/PVA electrolytic membranes as a function of the sulfonating agent added and SAC content. The total water content in N-PVA increased progressively to ~ 225% and 251% when the SA and CSA content reached the maximum loading content (4%), respectively. The water content found in the lower value with the pristine N-PVA was near 48%. In general, the swelling results exhibit a significant swelling behavior change with different sulfonating agent addition. This speculation is based on the polymer with a high ionic group possessing high water imbibing and high sorption capacity [63, 64] from which the amount of -SO3H groups in N/PVA membrane sulfonated with CSA is more than that sulfonated with SA. Thus, it possesses higher water uptake. This result is consistent with the obtained data by A.K. Sahu et al. [65]. In addition, Jimenez et al. [66] have proved related results for PVA membranes, and they found that the swelling ratio increased with the increment in the degree of sulfonation.
The influence of SAC filler incorporation on water uptake of sulfonated N/PVA-based composite membranes is shown in Fig. 5a. The total water content in sulfonated N/PVA membranes increased progressively until 318 and 410 for CSA and 4% SA, respectively, with the SAC content reaching the maximum loading content of 4%. As seen, water uptake increased with increasing amount of SAC regardless of the type of sulfonating agent (SA or CSA). These results correlated to that obtained by Fei-Xue et al. [67] for PVA/GO, and they found that the increase in the amount of GO led to an increased swelling ratio. On the other hand, varying sulfonating agents leads widely differences in the amount of water uptake using N-PVA-SAC membranes. Remarkably, the swelling ratio in N/PVA-SA-SAC is greater than that of N/PVA-CSA-SAC. This behavior can be explained by the degree of crosslinking which increased in the N/PVA-CSA-SAC membrane and produced a more rigid and compacted polymer structure with lower water uptake. This result is consistent with that reported by Parhi [68]. In addition, Chang et al. [69] have proved related results; they found that less crosslinked hydrogels exhibited a higher water uptake because highly crosslinked hydrogels could not sustain much imbibing water within their structure.
3.8 Methanol Uptake
In the case of DMFC, where methanol is used as a source of hydrogen, methanol uptake of the polyelectrolytic membrane must be lower to avoid fuel crossover [51]. Therefore, methanol uptake of pre-prepared N/PVA and modified N/PVA electrolytic membranes was determined, and the results are shown in Fig. 5b. These findings indicated that the percentage of methanol uptake decreased with an increase in the amount of SA, CSA, and SAC where the methanol uptake values reached 3.74 and 2.91 for SA- and CSA-based membranes, respectively. Likewise, blending SAC with sulfonated N/PVA-based membranes leads to a decrease in the methanol uptake of the resultant blend-based membranes reaching 2.76 and 2.03 for 4% SA-SAC and 4% CSA-SAC, respectively. This result proved that the modification process of N/PVA membranes has a more pronounced effect in decreasing the methanol holding capacity of the obtained membranes than the other non-modified N/PVA membranes consequently decreasing methanol crossover. On the contrary, the prepared membranes that possess higher water-holding capacity exhibited lower methanol uptake [31]. This observation can be attributed to the hydrophilic/hydrophobic behavior of polymer matrixes.
3.9 Gel Fraction
Figure 6A shows the gel fraction percent of N/PVA and the modified membranes. As seen, in the absence of SA (0% SA) content, the gel fraction found in the minimum value was about 65%, suggesting that N and PVA were not completely crosslinked, and gel strength was relatively low compared with others.
This behavior can be illustrated according to the strong polar groups in the polymer that can promote the adhesion of the polymer to many substrates through interactions with other polar chemical groups, thus facilitating the reaction between the polymer blends of PEM [70]. The interaction between N and PVA blend is only intramolecular forces which are relatively weak compared to other intermolecular forces such as the ester bond that formed between -COOH and -OH which founded in a small minor leading to low gel strength. This result is consistent with the obtained data by Alcântara for PVA blends [71]. With an increase in the amount of sulfonating agent in the polymer membrane from 1 to 3%, the gel fraction found in maximum value close to 92% and 95% for SA and CSA, respectively. This behavior is attributed to sulfonating agent content in N-PVA polymer membrane that afforded hydrogels with higher crosslinking than those from their initial blends which increased with an increase in the concentration of sulfonating agent to limit value at 3% SA. When the N-PVA are associated with -SO3H groups, the dual role of SA and CSA as a secondary crosslinker besides its basic role as a modifier agent was observed. This behavior is in agreement with data reported by Fei, et al. [72]. As shown in Scheme 1b, the acid makes an ionic bridge between polymer chains which leads to more crosslinking density as well as increasing gel fraction value. These results coincide with that obtained by Xu et al. [61]. At a high level of sulfonation (4%), the gel fraction of N-PVA starts to decrease. This performance might be attributed to the increase in acid to higher value leading to polymer chain deterioration. These results coincided with results obtained by Fahmy et al. for sulfonated PVA-HA hydrogel membrane [36].
Gel fraction of sulfonated N-PVA membrane as a different amount of SAC (0, 1, 2, and 4 wt %) addition is also presented in Fig. 6a. As shown, higher values of gel fraction were observed with the increase in SAC addition. The addition of SAC leads to a more crosslinked structure underscoring no more gel soluble in the medium. Crosslinking is greatly affected by the insertion of solid particles or platelets into a polymer matrix which leads to a highly dense structure, more interaction between polymer segments, and low polymer fractions [43, 73]. This behavior was observed for PI/SiO2 [74].
3.10 Ion Exchange Capacity (IEC)
It was suggested that the N/PVA modification with SA, CSA, or SAC is regarded as the primary factor to generate and improve IEC as well as proton conductivities [75, 76]. This might be due to the SA or CSA being the donor and the carrier of negatively charged groups (–SO3−H+) which are responsible for IEC and proton conductivity presence. The IEC measurements of N/PVA electrolytic membranes as a function of different concentrations of SA or CSA (0, 1, 2, 3, and 4%) are presented in Fig. 6b. IEC values range progressively from 0.12 to 1.72 and 0.12–2.03 Meq g−1, owing to the modification of N-PVA-based electrolytic membranes with SA and CSA, respectively, from 0 to 4%. These directories conclude that the IEC values increased with an increase in the introduced negative charges (i.e., –SO3− H+) of modifier agent [77] either SA or CSA within the N/PVA polymer matrix, while the N-PVA modified with CSA gave a relatively greater IEC than modified with SA (2.03–1.72 Meq g−1, respectively). The distinction of IEC results related to the type of modifier agent of N-PVA and the dependence of the electrochemical properties of membranes on the acid modifier agent and type, as lately discussed by Young et al. [78] and Fernandez et al. [79]. They found that the ionic conductivity and IEC of fully hydrated PVA-modified membranes increased significantly with the acid content increased. The obtained results of proton conductivity are typically consistent with the reported results of Rhim et al. [13]. They explored that the protonic conductivities and IEC of modified PVA membranes with SSA improved significantly, due to increasing number of charged negative ions (–SO3−H+ groups) which were responsible for holding and transporting protons (H+) through the polymeric membrane. Figure 6b also exhibits the correlation between the amount of SAC in sulfonated N/PVA membranes and IEC. The N-PVA-SAC exhibits high IEC in meq/g with an increment in SAC reached to 2.12 and 2.71 meq/g with SA and CSA, respectively, and found in lower value at 0% addition of SAC. This behavior can be illustrated in light of the fact that the increase in ionic groups in the polymer matrix leads to an increase in IEC [58]. Introducing SAC sheets into the sulfonated N-PVA polymer matrix increases the amount of –SO3H groups attached to the polymer matrix leading to more protons (H+) attached and transferred by the polyelectrolytic membrane. These results are close to that obtained by Hu et al. for PI/GO membranes [80]; in addition, Wang et al. [81] have provided related results for GO/Nafion.
3.11 Ionic Conductivity
Ionic conductivity or proton conductivity measurements of N/PVA, N/PVA-(4%) SA, and N/PVA-(4%) CSA electrolytic membranes as a function of different amounts of SAC (0, 1, 2, and 4%) are presented in Table 3. The N/PVA membrane possesses no ionic conductivity due to the absence of an ionic group attached to N or PVA polymeric chains which can hold and transport ions. At N/PVA-(4%) SA and N/PVA-(4%) CSA electrolytic membranes, the amount of ionic conductivity reached 0.059 and 0.061 S cm−1, respectively [39]. This performance is attributed to the insertion of (–SO3− H+) into N and PVA polymeric matrix during the sulfonation process. Sulfonic groups (–SO3H) can hold and facilitate proton transportation through the membrane due to the presence of a negative charge that can attract protons. With an increase in the amount of SAC from 0 to 4% in sulfonated N/PVA electrolytic membrane, the ionic conductivity increased and reached 0.076 and 0.087 S cm−1 at N/ PVA-4% SA-4% SAC and N/ PVA-4% CSA-4% SAC, respectively. This trend in behavior was the same as that observed for the IEC behavior (Fig. 6b) that increased with SAC concentration in N/PVA electrolytic membranes. The stated results are in conformity with that obtained by Yun-Sheng [82] for PVA/ Graphene.
3.11.1 Electrical Conductivity
Electrical resistivity is an important property for polymer electrolyte membrane fuel cell [83]. The prepared polymer electrolytic membranes do not possess any electrical conductivity at different impedance voltages. This is because the membranes are produced from poor conducting materials (Nylon6,6 and PVA) except SAC which have a quite electrical conductivity. The dispersion of SAC in a polymeric matrix is the key in which each SAC platelet is completely laminated by an electron-insulating polymeric matrix material (Nylon6,6 and PVA) that opposes the flow of electric current. In addition, the potential difference between two fuel cell parts is not enough to break down voltage and allow the electric current to pass through membranes [40]. This results in agreement with that obtained for GO or CCNT [18].
4 Conclusion
The polymer electrolytic membrane based on Nylon 6,6 and polyvinyl alcohol modified with sulfuric acid or chlorosulfonic acid and supported with sulfonated activated carbon was successfully synthesized using the casting technique. The structural and functional properties of prepared sulfonated N/PVA polyelectrolytic membranes and their blend-based membranes were characterized. The flexibility and stability are derived from the organic and inorganic components in the polymer membrane, respectively. Modification of pristine polymers using SA or CSA has achieved the purpose of which used to induce –SO3H groups that converted N-PVA as proton poor conductor into a good proton conductor and capacitor. The IEC value increased with the increase in sulfonation level of N/PVA and the proportion of SAC in the polymer blend. Ion exchange capacity and protonic conductivity reached their maximum 2.71 meq/g and 0.087 S/cm in the case of N/PVA-(4%) CSA-(4%) SAC blend-based membranes. It was thus noted that swelling behavior has a great influence on N/PVA electrolytic membranes conductivity; accordingly, certain water sorption facilitates and accelerates conducting the protons through the membranes. In addition, the incorporation of SAC fillers significantly improved the membrane properties as mechanical stability and lowered methanol uptake made the membrane have good barrier properties to fuel crossover. It was demonstrated that N-PVA-SAC membranes possess acceptable electrochemical performance, e.g., IEC, ionic conductivity, and mechanical stability values at ambient temperature. Finally, the novel N/PVA-SAC polyelectrolytic membranes show high potential for further low-cost DMFC applications.
References
Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T.: Activity benchmarks and requirements for pt, pt-alloy, and non-pt oxygen reduction catalysts for pemfcs. Appl. Catal. B 56, 9–35 (2005)
Kumar A.; Singh T.; Singh S.; Liu Y.: A comprehensive review of fuel cell and its types. (2013).
Litster, S.; McLean, G.: Pem fuel cell electrodes. J. Power Sources 130, 61–76 (2004)
Nasirinezhad, M.; Ghaffarian, S.R.; Tohidian, M.: Eco-friendly polyelectrolyte nanocomposite membranes based on chitosan and sulfonated chitin nanowhiskers for fuel cell applications. Iran. Polym. J. 30, 355–367 (2021)
Realpe, A.; Barrios, K.; Acevedo, M.: Proton exchange membranes with titanium dioxide prepared by sulfonation of rubber. Int. J. Appl. Eng. Res. 10, 5023–5030 (2015)
Munakata, H.; Yamamoto, D.; Kanamura, K.: Three-dimensionally ordered macroporous polyimide composite membrane with controlled pore size for direct methanol fuel cells. J. Power Sources 178, 596–602 (2008)
Lufrano, F.; Baglio, V.; Staiti, P.; Antonucci, V.: Polymer electrolytes based on sulfonated polysulfone for direct methanol fuel cells. J. Power Sources 179, 34–41 (2008)
Zhang, X.; Tay, S.W.; Hong, L.; Liu, Z.: In situ implantation of polyposs blocks in nafion® matrix to promote its performance in direct methanol fuel cell. J. Membr. Sci. 320, 310–318 (2008)
Oliveira, P.N.D.; Mebdes, A.M.M.: Preparation and characterization of an eco-friendly polymer electrolyte membrane (pem) based in a blend of sulphonated poly (vinyl alcohol)/chitosan mechanically stabilised by nylon. Mater. Res. 19, 954–962 (2016)
Tsai, C.-E.; Lin, C.-W.; Hwang, B.-J.: A novel crosslinking strategy for preparing poly (vinyl alcohol)-based proton-conducting membranes with high sulfonation. J. Power Sources 195, 2166–2173 (2010)
Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C.: A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22, 1259–1279 (2016)
Quintana, D.; Baca, E.; Mosquera, E.; Vargas, R.; Diosa, J.: Improving the ionic conductivity in nanostructured membranes based on poly (vinyl alcohol)(pva), chitosan (cs), phosphoric acid (H3PO4), and niobium oxide (Nb2O5). Ionics 25(3), 1131–1136 (2019)
Rhim, J.-W.; Park, H.B.; Lee, C.-S.; Jun, J.-H.; Kim, D.S.; Lee, Y.M.: Crosslinked poly (vinyl alcohol) membranes containing sulfonic acid group: proton and methanol transport through membranes. J. Membr. Sci. 238, 143–151 (2004)
Zhiwei, W.; Hao, Z.; Qiang, C.; Sumei, Z.; Feng, Y.; Jian, K.; Jinyao, C.; Ya, C.; Ming, X.: Preparation and characterization of pva proton exchange membranes containing phosphonic acid groups for direct methanol fuel cell applications. J. Polym. Res. 26, 1–10 (2019)
Panero, S.; Fiorenza, P.; Navarra, M.; Romanowska, J.; Scrosati, B.: Silica-added, composite poly (vinyl alcohol) membranes for fuel cell application. J. Electrochem. Soc. 152, A2400 (2005)
Son, J.H.; Kang, Y.S.; Won, J.: Poly (vinyl alcohol)-based polymer electrolyte membranes containing polyrotaxane. J. Membr. Sci. 281, 345–350 (2006)
Xu, J.; Cheng, L.; Zhang, Z.; Zhang, L.; Xiong, C.; Huang, W.; Xie, Y.; Yang, L.: Highly exfoliated montmorillonite clay reinforced thermoplastic polyurethane elastomer: In situ preparation and efficient strengthening. RSC Adv. 9, 8184–8196 (2019)
Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A.: Composite polymers development and application for polymer electrolyte membrane technologies—a review. Molecules 25, 1712 (2020)
Tseng, C.-Y.; Ye, Y.-S.; Kao, K.-Y.; Joseph, J.; Shen, W.-C.; Rick, J.; Hwang, B.-J.: Interpenetrating network-forming sulfonated poly (vinyl alcohol) proton exchange membranes for direct methanol fuel cell applications. Int. J. Hydrogen Energy 36, 11936–11945 (2011)
Gunduz N.; McGrath J.: Synthesis and characterization of sulfonated polyimides. in 'Abstracts of Papers of the American Chemical Society. 219: U373-U373 (2000).
Einsla, B.R.; Kim, Y.S.; Hickner, M.A.; Hong, Y.-T.; Hill, M.L.; Pivovar, B.S.; McGrath, J.E.: Sulfonated naphthalene dianhydride based polyimide copolymers for proton-exchange-membrane fuel cells: Ii. Membrane properties and fuel cell performance. J. Membr. Sci. 255, 141–148 (2005)
Bai, H.; Ho, W.W.: New poly (ethylene oxide) soft segment-containing sulfonated polyimide copolymers for high temperature proton-exchange membrane fuel cells. J. Membr. Sci. 313, 75–85 (2008)
Jamil, A.; Ching, O.P.; Shariff, A.B.: Current status and future prospect of polymer-layered silicate mixed-matrix membranes for co2/ch4 separation. Chem. Eng. Technol. 39, 1393–1405 (2016)
Hocker, S.; Hudson-Smith, N.; Schniepp, H.C.; Kranbuehl, D.E.: Enhancing polyimide’s water barrier properties through addition of functionalized graphene oxide. Polymer 93, 23–29 (2016)
Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S.: Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924 (2010)
Geim, A.K.; Novoselov, K.S.: The rise of graphene. In: Rodgers, P. (Ed.) Nanoscience and Technology: A Collection of Reviews from Nature Journals, pp. 11–19. Co-Published with Macmillan Publishers Ltd, UK (2009). https://doi.org/10.1142/9789814287005_0002
Kim, H.; Miura, Y.; Macosko, C.W.: Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 22, 3441–3450 (2010)
Peres, N.: The transport properties of graphene. J. Phys.: Condens. Matter 21, 323201 (2009)
Layek, R.K.; Nandi, A.K.: A review on synthesis and properties of polymer functionalized graphene. Polymer 54, 5087–5103 (2013)
Bermejo, B.; Fraga, A.C.; Sousa-Aguiar, E.: The role of sulfonated activated carbons as catalysts for the hydrolysis of cellobiose. Braz. J. Chem. Eng. 36, 309–315 (2019)
Abu-Saied, M.; El-Desouky, E.; Soliman, E.; Abd, E.-N.: Novel sulphonated poly (vinyl chloride)/poly (2-acrylamido-2-methylpropane sulphonic acid) blends-based polyelectrolyte membranes for direct methanol fuel cells. Polym. Testing 89, 106604 (2020)
Hasan, M.; Banerjee, A.N.; Lee, M.: Enhanced thermo-optical performance and high bet surface area of graphene@ pvc nanocomposite fibers prepared by simple facile deposition technique: N2 adsorption study. J. Ind. Eng. Chem. 21, 828–834 (2015)
Yang, C.-C.; Lue, S.J.; Shih, J.-Y.: A novel organic/inorganic polymer membrane based on poly (vinyl alcohol)/poly (2-acrylamido-2-methyl-1-propanesulfonic acid/3-glycidyloxypropyl trimethoxysilane polymer electrolyte membrane for direct methanol fuel cells. J. Power Sources 196, 4458–4467 (2011)
Abu-Saied, M.; Soliman, E.; Al, D.E.: Development of proton exchange membranes based on chitosan blended with poly (2-acrylamido-2-methylpropane sulfonic acid) for fuel cells applications. Mater. Today Commun. 25, 101536 (2020)
Shayestehfar, S.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.-S.: Physical and mechanical properties of nylon 6/titanium dioxide micro and nano-composite multifilament yarns. J. Eng. Fibers Fabr. 9, 155892501400900320 (2014)
Fahmy, A.; Abu-Saied, M.; Morgan, N.; Qutop, W.; Abdelbary, H.; Salama, T.: Surface modification of polyvinyl chloride by polyacrylic acid graft as a polyelectrolyte membrane using ar plasma. Turk. J. Chem. 43, 1686–1696 (2019)
Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P.: Preparation and properties of polyvinyl alcohol/n–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohyd. Polym. 261, 117875 (2021)
Fahmy, A.; Saied, M.A.; Morgan, N.; Qutop, W.; Abdelbary, H.; El-Bahy, S.M.; Schönhals, A.; Friedrich, J.F.: Modified polyvinyl chloride membrane grafted with an ultra-thin polystyrene film: structure and electrochemical properties. J. Mater. Res. Technol. 12, 2273–2284 (2021)
Kakati, N.; Maiti, J.; Das, G.; Lee, S.H.; Yoon, Y.S.: An approach of balancing the ionic conductivity and mechanical properties of pva based nanocomposite membrane for dmfc by various crosslinking agents with ionic liquid. Int. J. Hydrogen Energy 40, 7114–7123 (2015)
Bazli, L.; Eskandarinezhad, S.; Kakur, N.; Ramachandran, V.; Bacigalupe, A.; Mansilla, M.; Escobar, M.: Electrical properties of polymer blend composites based on silicone rubber/epdm/clay for high voltage insulators. J. Compos. Compd. 3, 18–24 (2021)
Leonor, I.; Kim, H.-M.; Balas, F.; Kawashita, M.; Reis, R.; Kokubo, T.; Nakamura, T.: Functionalization of different polymers with sulfonic groups as a way to coat them with a biomimetic apatite layer. J. Mater. Sci. - Mater. Med. 18, 1923–1930 (2007)
Reddy B. S.; Gnanasekaran D.: Structure-gas transport property relationships of poly (dimethylsiloxane-urethane) nanocomposite membranes. in 'Book title' (eds.: Intech–Publishing Croatia, Vol. 195–226 (2011).
Diyuk, V.E.; Mariychuk, R.T.; Lisnyak, V.V.: Functionalization of activated carbon surface with sulfonated styrene as a facile route for solid acids preparation. Mater. Chem. Phys. 184, 138–145 (2016)
Hara, M.; Yoshida, T.; Takagaki, A.; Takata, T.; Kondo, J.N.; Hayashi, S.; Domen, K.: A carbon material as a strong protonic acid. Angew. Chem. 116, 3015–3018 (2004)
Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y.: Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 15, 7188–7196 (2010)
Onda, A.; Ochi, T.; Yanagisawa, K.: Hydrolysis of cellulose selectively into glucose over sulfonated activated-carbon catalyst under hydrothermal conditions. Top. Catal. 52, 801–807 (2009)
Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R.: Development of poly (vinyl alcohol)-based polymers as proton exchange membranes and challenges in fuel cell application: A review. Polym. Rev. 60, 171–202 (2020)
Fahmy, A.; Friedrich, J.; Poncin-Epaillard, F.; Debarnot, D.: Plasma polymerized allyl alcohol/o2 thin films embedded with silver nanoparticles. Thin Solid Films 616, 339–347 (2016)
Salehi, E.; Farahani, A.: Macroporous chitosan/polyvinyl alcohol composite adsorbents based on activated carbon substrate. J. Porous Mater. 24, 1197–1207 (2017)
Abu-Saied, M.; Elzatahry, A.; El-Khatib, K.; Hassan, E.; El-Sabbah, M.; Drioli, E.; Mohy, E.M.: Preparation and characterization of novel grafted cellophane-phosphoric acid-doped membranes for proton exchange membrane fuel-cell applications. J. Appl. Polym. Sci. 123, 3710–3724 (2012)
Abu-Saied, M.; Soliman, E.A.; Abualnaj, K.M.; El Desouky, E.: Highly conductive polyelectrolyte membranes poly (vinyl alcohol)/poly (2-acrylamido-2-methyl propane sulfonic acid)(pva/pamps) for fuel cell application. Polymers 13, 2638 (2021)
Badawy, S.M.: Green synthesis of dual-surface nanocomposite films using tollen’s method. Green Process. Synth. 3, 463–469 (2014)
Fahmy, A.; Mohamed, T.A.; Abu-Saied, M.; Helaly, H.; El-Dossoki, F.: Structure/property relationship of polyvinyl alcohol/dimethoxydimethylsilane composite membrane: Experimental and theoretical studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 228, 117810 (2020)
Stevens, R.W., Jr.; Siriwardane, R.V.; Logan, J.: In situ fourier transform infrared (ftir) investigation of co2 adsorption onto zeolite materials. Energy Fuels 22, 3070–3079 (2008)
Baudoux, J.; Lefebvre, P.; Legay, R.; Lasne, M.-C.; Rouden, J.: Environmentally benign metal-free decarboxylative aldol and mannich reactions. Green Chem. 12, 252–259 (2010)
Taha, T.H.; Elnouby, M.S.; Abu-Saied, M.; Alamri, S.: The green exfoliation of graphite waste and its suitability for biosensor applications. RSC Adv. 10, 9347–9355 (2020)
Yvon H. J.: Raman spectroscopy for analysis and monitoring. nd). Retrieved from http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Raman/bands.pdf , (2017).
Abu-Saied, M.; Taha, T.H.; El-Deeb, N.M.; Hafez, E.E.: Polyvinyl alcohol/sodium alginate integrated silver nanoparticles as probable solution for decontamination of microbes contaminated water. Int. J. Biol. Macromol. 107, 1773–1781 (2018)
Budai A.; Zimmerman A.; Cowie A.; Webber J.; Singh B.; Glaser B.; Masiello C.; Andersson D.; Shields F.; Lehmann J.: Biochar carbon stability test method: An assessment of methods to determine biochar carbon stability. Int. Biochar Initiative, 1–10 (2013).
Mazurkiewicz-Pawlicka, M.; Nowak, M.; Malolepszy, A.; Witowski, A.; Wasik, D.; Hu, Y.; Stobinski, L.: Graphene oxide with controlled content of oxygen groups as a filler for polymer composites used for infrared radiation shielding. Nanomaterials 10, 32 (2020)
Xu, L.; Huang, Y.-A.; Zhu, Q.-J.; Ye, C.: Chitosan in molecularly-imprinted polymers: current and future prospects. Int. J. Mol. Sci. 16, 18328–18347 (2015)
Bhattacharya, M.: Polymer nanocomposites—a comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 9, 262 (2016)
Brown, M.B.; Jones, S.A.: Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 19, 308–318 (2005)
Tang, Q.; Cai, H.; Yuan, S.; Wang, X.; Yuan, W.: Enhanced proton conductivity from phosphoric acid-imbibed crosslinked 3d polyacrylamide frameworks for high-temperature proton exchange membranes. Int. J. Hydrogen Energy 38, 1016–1026 (2013)
Sahu, A.; Selvarani, G.; Bhat, S.; Pitchumani, S.; Sridhar, P.; Shukla, A.; Narayanan, N.; Banerjee, A.; Chandrakumar, N.: Effect of varying poly (styrene sulfonic acid) content in poly (vinyl alcohol)–poly (styrene sulfonic acid) blend membrane and its ramification in hydrogen–oxygen polymer electrolyte fuel cells. J. Membr. Sci. 319, 298–305 (2008)
Jimenez, A.; Gedeón, C.P.; Castro, A.G.: Effect of the sulfonation on proton exchange membrane synthesized from polyvinyl alcohol for fuel cell. Int. J. Appl. Eng. Res. 13, 12616–12619 (2018)
Xue, F.; He, X.; Cai, S.; Nie, J.; Shi, Z.; Wang, X.: Synergistic effect of graphene oxide and sodium carboxymethylcellulose on the properties of poly (vinyl alcohol) hydrogels. J. Appl. Polym. Sci. 136, 47644 (2019)
Parhi, R.: Cross-linked hydrogel for pharmaceutical applications: A review. Advanced pharmaceutical bulletin 7, 515–530 (2017)
Chang, C.; Lue, A.; Zhang, L.: Effects of crosslinking methods on structure and properties of cellulose/pva hydrogels. Macromol. Chem. Phys. 209, 1266–1273 (2008)
Zheng, P.; Liu, Q.; Li, Z.; Wang, D.; Liu, X.: Effect of crosslinking degree on sulfonated poly (aryl ether nitrile) s as candidates for proton exchange membranes. Polymers 11, 964 (2019)
Alcântara, M.; Brant, A.; Giannini, D.; Pessoa, J.; Andrade, A.; Riella, H.; Lugão, A.: Influence of dissolution processing of pva blends on the characteristics of their hydrogels synthesized by radiation—part i: Gel fraction, swelling, and mechanical properties. Radiat. Phys. Chem. 81, 1465–1470 (2012)
Fei, H.; Yang, C.; Bao, H.; Wang, G.: Flexible all-solid-state supercapacitors based on graphene/carbon black nanoparticle film electrodes and cross-linked poly (vinyl alcohol)–h2so4 porous gel electrolytes. J. Power Sources 266, 488–495 (2014)
Jia, R.; Ren, J.; Liu, X.; Lu, G.; Wang, Y.: Design and synthesis of sulfonated carbons with amphiphilic properties. J. Mater. Chem. A 2, 11195–11201 (2014)
Soroko, I.; Livingston, A.: Impact of tio2 nanoparticles on morphology and performance of crosslinked polyimide organic solvent nanofiltration (osn) membranes. J. Membr. Sci. 343, 189–198 (2009)
Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.: Polypropylene/montmorillonite nanocomposites. Review of the synthetic routes and materials properties. Chem. Mater. 13, 3516–3523 (2001)
Li, H.; Zhang, G.; Wu, J.; Zhao, C.; Zhang, Y.; Shao, K.; Han, M.; Lin, H.; Zhu, J.; Na, H.: A novel sulfonated poly (ether ether ketone) and cross-linked membranes for fuel cells. J. Power Sources 195, 6443–6449 (2010)
Zhang, Y.; Wan, Y.; Pan, G.; Shi, H.; Yan, H.; Xu, J.; Guo, M.; Wang, Z.; Liu, Y.: Surface modification of polyamide reverse osmosis membrane with sulfonated polyvinyl alcohol for antifouling. Appl. Surf. Sci. 419, 177–187 (2017)
Chang, Y.W.; Wang, E.; Shin, G.; Han, J.E.; Mather, P.T.: Poly (vinyl alcohol)(pva)/sulfonated polyhedral oligosilsesquioxane (sposs) hybrid membranes for direct methanol fuel cell applications. Polym. Adv. Technol. 18, 535–543 (2007)
Fernandez, M.; Castillo, J.; Bedoya, F.; Diosa, J.; Vargas, R.: Dependence of the mechanical and electrical properties on the acid content in pva+ H3PO2+ H2O membranes. Revista mexicana de física 60, 249–252 (2014)
Hu, R.; He, Y.; Zhang, C.; Zhang, R.; Li, J.; Zhu, H.: Graphene oxide-embedded polyamide nanofiltration membranes for selective ion separation. J. Mater. Chem. A 5, 25632–25640 (2017)
Wang, L.; Kang, J.; Nam, J.-D.; Suhr, J.; Prasad, A.K.; Advani, S.G.: Composite membrane based on graphene oxide sheets and nafion for polymer electrolyte membrane fuel cells. ECS Electrochem. Lett. 4, F1 (2015)
Ye, Y.-S.; Cheng, M.-Y.; Xie, X.-L.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J.: Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J. Power Sources 239, 424–432 (2013)
Jamil, A.; Rafiq, S.; Iqbal, T.; Khan, H.A.A.; Khan, H.M.; Azeem, B.; Mustafa, M.; Hanbazazah, A.S.: Current status and future perspectives of proton exchange membranes for hydrogen fuel cells. Chemosphere 303, 1352046 (2022)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, A., Saied, M.A., Naser, A. et al. Synthesis and Characterization of Nylon 6,6-Polyvinyl Alcohol-Based Polyelectrolytic Membrane. Arab J Sci Eng 48, 8941–8956 (2023). https://doi.org/10.1007/s13369-022-07537-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07537-3