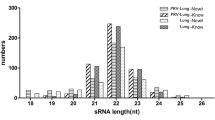
Abstract
Pseudorabies virus (PRV), an alpha herpesvirus can enter the mammalian nervous system, causing Aujezsky’s disease. Previous studies have reported an alteration of microRNA (miRNA) expression levels during PRV infections. However, knowledge regarding miRNA response in nervous cells to PRV infection is still unknown. To address this issue, small RNA libraries from infected and uninfected mouse neuroblastoma cells were assessed after Illumina deep sequencing. A total of eight viral miRNA were identified, and ten host miRNAs showed significantly different expression upon PRV infection. Among these, five were analyzed by stem-loop RT-qPCR, which confirmed the above data. Interestingly, these viral miRNAs were mainly found in the large latency transcript region of PRV, and predicted to target a variety of genes, forming a complicated regulatory network. Moreover, ten cellular miRNAs were expressed differently upon PRV infection, including nine upregulated and one downregulated miRNAs. Host targets of these miRNAs obtained by bioinformatics analysis belonged to large signaling networks, mainly encompassing calcium signaling pathway, cAMP signaling pathway, MAPK signaling pathway, and other nervous-associated pathways. These findings further highlighted miRNA features in nervous cells after PRV infection and contributed to unveil the underlying mechanisms of neurotropism as well as the neuropathogenesis of PRV.





Similar content being viewed by others
References
An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Tong GZ (2013) Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis 19:1749–1755
Anselmo A, Flori L, Jaffrezic F, Rutigliano T, Cecere M, Cortes-Perez N, Giuffra E (2011) Co-expression of host and viral microRNAs in porcine dendritic cells infected by the pseudorabies virus. PLoS One 6:e17374
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 57:289–300
Besecker MI, Harden ME, Li G, Wang XJ, Griffiths A (2009) Discovery of herpes B virus-encoded microRNAs. J Virol 83:3413–3416
Boss IW, Plaisance KB, Renne R (2009) Role of virus-encoded microRNAs in herpesvirus biology. Trends Microbiol 17(12):544–553
Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR (2005) Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci U S A 102:5570–5575
Cai Y, Zhu L, Zhou Y, Liu X, Liu X, Li X, Lang Q, Qiao X, Xu Z (2015) Identification and analysis of differentially-expressed microRNAs in Japanese encephalitis virus-infected PK-15 cells with deep sequencing. Int J Mol Sci 16:2204–2219
Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, Flemington EK (2008) Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol 82:1946–1958
Fuchs W, Granzow H, Klupp BG, Kopp M, Mettenleiter TC (2002) The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J Virol 76:6729–6742
Grey F (2015) Role of microRNAs in herpesvirus latency and persistence. J Gen Virol 96:739–751
Grey F, Antoniewicz A, Allen E, Saugstad J, McShea A, Carrington JC, Nelson J (2005) Identification and characterization of human cytomegalovirus-encoded microRNAs. J Virol 79:12095–12099
Grey F, Meyers H, White EA, Spector DH, Nelson J (2007) A human cytomegalovirus-encoded microRNA regulates expression of multiple viral genes involved in replication. PLoS Pathog 3:e163
Grey F, Hook L, Nelson J (2008) The functions of herpesvirus-encoded microRNAs. Med Microbiol Immunol 197:261–267
Hicks J, Liu HC (2013) Involvement of eukaryotic small RNA pathways in host defense and viral pathogenesis. Viruses 5:2659–2678
Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ (2009) HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. Neuroreport 20:1500–1505
Hou J, Wang P, Lin L, Liu X, Ma F, An H, Cao X (2009) MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 183:2150–2158
Hou ZH, Han QJ, Zhang C, Tian ZG, Zhang J (2014) miR146a impairs the IFN-induced anti-HBV immune response by downregulating STAT1 in hepatocytes. Liver Int 34:58–68
Huang J, Ma G, Fu L, Jia H, Zhu M, Li X, Zhao S (2014) Pseudorabies viral replication is inhibited by a novel target of miR-21. Virology 456:319–328
Kincaid RP, Sullivan CS (2012) Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog 8:e1003018
Klupp BG, Hengartner CJ, Mettenleiter TC, Enquist LW (2004) Complete, annotated sequence of the pseudorabies virus genome. J Virol 78:424–440
Labbaye C, Testa U (2012) The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J Hematol Onco l5:13
Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Voinnet O (2005) A cellular microRNA mediates antiviral defense in human cells. Science 308:557–560
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S, An T (2013) miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett 339:260–269
Li W, Chang J, Wang S, Liu X, Peng J, Huang D, Sun M, Chen Z, Zhang W, Guo W, Li J (2015a) miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget 6:24448–24462
Li Y, Chang H, Yang X, Zhao Y, Chen L, Wang X, Zhao J (2015b) Antiviral activity of porcine interferon regulatory factor 1 against swine viruses in cell culture. Viruses 7:5908–5918
Lin X, Liang D, He Z, Deng Q, Robertson ES, Lan K (2011) miR-K12-7-5p encoded by Kaposi’s sarcoma-associated herpesvirus stabilizes the latent state by targeting viral ORF50/RTA. PLoS One 6:e16224
Liu J, Xu J, Li H, Sun C, Yu L, Li Y, Wen Y (2015) miR-146b-5p functions as a tumor suppressor by targeting TRAF6 and predicts the prognosis of human gliomas. Oncotarget 6:29129–29142
Liu F, Zheng H, Tong W, Li GX, Tian Q, Liang C, Tong GZ (2016) Identification and analysis of novel viral and host dysregulated microRNAs in variant pseudorabies virus-infected PK15 cells. PLoS One 11:e0151546
Mahjoub N, Dhorne-Pollet S, Fuchs W, Ahanda MLE, Lange E, Klupp B, Giuffra E (2015) A 2.5-kilobase deletion containing a cluster of nine microRNAs in the latency-associated-transcript locus of the pseudorabies virus affects the host response of porcine trigeminal ganglia during established latency. J Virol 89:428–442
Mellencamp MW, O'brien PC, Stevenson JR (1991) Pseudorabies virus-induced suppression of major histocompatibility complex class I antigen expression. J Virol 65:3365–3368
Mulder WAM, Pol JMA, Gruys E, Jacobs L, De Jong MCM, Peeters BPH, Kimman TG (1997) Pseudorabies virus infections in pigs: role of viral proteins in virulence, pathogenesis and transmission. Vet Res 28:1–17
Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Das SC (2007) Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 27:123–134
Perng GC, Jones C (2010) Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdiscip Perspect Infect Dis 2010:262415
Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, Tuschl T (2004) Identification of virus-encoded microRNAs. Science 304:734–736
Piedade D, Azevedo-Pereira JM (2016) The role of microRNAs in the pathogenesis of herpesvirus infection. Viruses 8:156
Pivovarova NB, Andrews SB (2010) Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J 277:3622–3636
Pomeranz LE, Reynolds AE, Hengartner CJ (2005) Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69(3):462–500
Punj V, Matta H, Schamus S, Tamewitz A, Anyang B, Chaudhary PM (2010) Kaposi's sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 suppresses CXCR4 expression by upregulating miR-146a. Oncogene 29:1835–1844
Saba R, Sorensen DL, Booth SA (2014) MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front Immunol 5:578
Singh Y, Kaul V, Mehra A, Chatterjee S, Tousif S, Dwivedi VP, Suar M, Van Kaer L, Bishai WR, Das G (2013) Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J Biol Chem 288:5056–5061
Stewart CR, Marsh GA, Jenkins KA, Gantier MP, Tizard ML, Middleton D, Deffrasnes C (2013) Promotion of Hendra virus replication by microRNA 146a. J Virol 87:3782–3791
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103:12481–12486
Tang S, Patel A, Krause PR (2009) Novel less-abundant viral microRNAs encoded by herpes simplex virus 2 latency-associated transcript and their roles in regulating ICP34.5 and ICP0 mRNAs. J Virol 83:1433–1442
Tang S, Bertke AS, Patel A, Margolis TP, Krause PR (2011) Herpes simplex virus 2 microRNA miR-H6 is a novel latency-associated transcript-associated microRNA, but reduction of its expression does not influence the establishment of viral latency or the recurrence phenotype. J Virol 85:4501–4509
Timoneda O, Núñez-Hernández F, Balcells I, Muñoz M, Castelló A, Vera G, Rosell R (2014) The role of viral and host microRNAs in the Aujeszky’s disease virus during the infection process. PLoS One 9:e86965
Tong W, Liu F, Zheng H, Liang C, Zhou YJ, Jiang YF, Tong GZ (2015) Emergence of a pseudorabies virus variant with increased virulence to piglets. Vet Microbiol 181:236–240
Tong W, Li G, Liang C, Liu F, Tian Q, Cao Y, Li L, Zheng X, Zheng H, Tong GZ (2016) A live, attenuated pseudorabies virus strain JS-2012 deleted for gE/gI protects against both classical and emerging strains. Antivir Res 130:110–117
Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR (2008) MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–783
Van Opdenbosch N, Van den Broeke C, De Regge N, Tabarés E, Favoreel HW (2012) The IE180 protein of pseudorabies virus suppresses phosphorylation of translation initiation factor eIF2α. J Virol 86:7235–7240
Varkonyi-Gasic E, Hellens RP (2011) Quantitative stem-loop RT-PCR for detection of microRNAs. Methods Mol Biol 744:145–157
Wang LL, Huang Y, Wang G, Chen SD (2012) The potential role of microRNA-146 in Alzheimer’s disease: biomarker or therapeutic target. Med Hypotheses 78:398–401
Wang X, Diao C, Yang X, Yang Z, Liu M, Li X, Tang H (2016) ICP4-induced miR-101 attenuates HSV-1 replication. Sci Rep 6:23205
Wu YQ, Chen DJ, He HB, Chen DS, Chen LL, Chen HC, Liu ZF (2012) Pseudorabies virus infected porcine epithelial cell line generates a diverse set of host microRNAs and a special cluster of viral microRNAs. PLoS One 7(1):e30988
Wu S, He L, Li Y, Wang T, Feng L, Jiang L, Huang X (2013) miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J Infect 67:329–341
Wu BW, Engel EA, Enquist LW (2014) Characterization of a replication-incompetent pseudorabies virus mutant lacking the sole immediate early gene IE180. MBio 5:e01850
Xiang M, Birkbak NJ, Vafaizadeh V, Walker S, Yeh JE, Liu S, Richardson AL (2014) STAT3 induction of miR-146b forms a feedback loop to inhibit the NF-κB to IL-6 signaling axis and STAT3-driven cancer phenotypes. Sci Signal 7(310):ra11
Ye X, Luo H, Chen Y, Wu Q, Xiong Y, Zhu J, Diao Y, Wu Z, Miao J, Wan J (2015a) MicroRNAs 99b-5p/100-5p regulated by endoplasmic reticulum stress are involved in Abeta-induced pathologies. Front Aging Neurosci 7:210
Ye C, Zhang QZ, Tian ZJ, Zheng H, Zhao K, Liu F, Shi M (2015b) Genomic characterization of emergent pseudorabies virus in China reveals marked sequence divergence: evidence for the existence of two major genotypes. Virology 483:32–43
Zheng SQ, Li YX, Zhang Y, Li X, Tang H (2011) MiR-101 regulates HSV-1 replication by targeting ATP5B. Antivir Res 89:219–226
Acknowledgements
The present study was funded by the National Natural Science Foundation of China (31272567), the Program for Innovative Research Team (in Science and Technology) at the University of Henan (14IRTSTHN015), and the Science and Technology Innovation Talent Support Plan of Colleges and Universities in Henan Province (14HASTIT022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Yongtao Li and Guanmin Zheng contributed equally to this work.
Electronic supplementary material
Supplementary file 1
Predicted target genes of differentially expressed host miRNAs at 4 hpi. (XLS 1071 kb)
Supplementary file 2
Predicted target genes of differentially expressed host miRNAs at 28 hpi. (XLS 1194 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Zheng, G., Zhang, Y. et al. MicroRNA analysis in mouse neuro-2a cells after pseudorabies virus infection. J. Neurovirol. 23, 430–440 (2017). https://doi.org/10.1007/s13365-016-0511-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-016-0511-y