Abstract
Following nineteenth-century declines, polecats Mustela putorius are recolonising Great Britain. Polecat diet relates to two potential risks to recovery. First, rabbits Oryctolagus cuniculus, which are important prey for polecats, have experienced extreme population fluctuations, with near extirpation due to myxomatosis in the 1950s, recovery in 1960s–1990s and declines in 1990s–2010s. Second, polecats are secondarily exposed to anticoagulant rodenticides by eating contaminated rodents, and the frequency of polecat exposure to rodenticides is increasing. We analysed stomach contents from 99 polecats collected in 2012–2016 and compared results with earlier studies. Lagomorphs were the most abundant prey (66% frequency of occurrence, 95% confidence interval 53–74%), followed by other mammals (12%, 4–18%), amphibians (10%, 3–16%) and birds (7%, 1–13%). Diet varied seasonally; lagomorph occurrence was highest in spring and summer and lowest in autumn. Dietary niche breadth was greater in the 1960s, when rabbits were scarce, than in other decades, but did not differ between the 1990s and 2010s, indicating that diets have not diversified with recent rabbit declines. This may be because rabbit abundance is not yet low enough to cause dietary diversification or because polecats were collected in areas where rabbits were still abundant. Rodents did not increase in diet between the 1990s and 2010s and still occur with < 10% frequency, indicating that rodents need not contribute much to diet to expose polecats to rodenticides. This potentially limits the effectiveness of management actions designed to minimise polecat exposure to contaminated rodent prey.
Similar content being viewed by others
Introduction
Successfully colonizing species often demonstrate ecological flexibility during the process of population establishment and expansion (Rosecchi et al. 2001; Lee and Gelembiuk 2008). Some species have flexible foraging strategies that allow them to exploit variation in resource availability across temporal and spatial scales (Zhou et al. 2011). The European polecat Mustela putorius is a medium-sized mustelid carnivoran that is currently recolonising its former range in Great Britain, following catastrophic declines, mostly in the nineteenth century (Langley and Yalden 1977; Sainsbury et al. 2019). A significant element of this period of range expansion has coincided with extreme fluctuations in populations of rabbits Oryctolagus cuniculus (Sumption and Flowerdew 1985; Aebischer et al. 2011; Harris et al. 2018; Massimino et al. 2019), which are a major source of food for polecats in Great Britain (Birks and Kitchener 1999). It is unknown whether polecats in Britain may have altered their feeding strategy in response to recent rabbit declines and whether these declines may have impacted on their continued recolonisation.
Optimal foraging theory predicts that animals will maximise their net energy intake (MacArthur and Pianka 1966; Pyke et al. 1977; Perry and Pianka 1997), which may be maximised via different foraging strategies. For instance, specialist predators have narrow dietary niches and will forage for specific prey species, independent of their availability, whereas generalists have comparatively large dietary niches and consume prey in proportion to their availability (Futuyma and Moreno 1988; Amundsen 1995). Polecats eat a wide variety of food items across their European range and are usually described as generalist predators (Erlinge 1986; Lodé 1995; Baghli et al. 2002; Santos et al. 2009; Malecha and Antczak 2013). Rodents and amphibians are common food items in all regions (Lodé 1997). While polecats exhibit dietary diversity across their range, there is some evidence of regional specialisation. For example, polecats specialise on rabbits in the Mediterranean (Santos et al. 2009) and on amphibians in Switzerland (Weber 1989a) and Poland (Jędrzejewski et al. 1993; Jędrzejewska and Jędrzejewska 1998). In Białowieża National Park, Poland, frogs comprised 60% and 90% of polecat total food biomass in summer and winter, respectively, and although polecats ate rodents, this was only when frogs were not available (Jędrzejewska and Jędrzejewska 1998). In this example, polecats exhibited a very narrow dietary niche and clear preferential selection for frogs. In other localities, it has been found that impressions of specialisation by polecats simply reflect the local abundance of a given prey (Lodé 1995). Whilst more recent studies in Britain have found that polecats predominantly eat lagomorphs (Blandford 1986; Birks and Kitchener 1999), in periods when rabbit populations were severely reduced due to disease outbreaks, notably during the 1950s and 1960s due to myxomatosis (Sumption and Flowerdew 1985), mammals comprised a much smaller proportion of polecat diet (Walton 1968). There is also evidence of seasonal consumption of rodents (including brown rats Rattus norvegicus and field voles Microtus agrestis) particularly in the winter months (Birks 1998; Birks and Kitchener 1999). A flexible foraging strategy is thought to allow polecats to occupy and exploit diverse habitats, such as lowland, grassland, farmland and riparian habitats (Blandford 1987) and may play an important role in enabling population persistence (Lodé 1997).
When rabbit populations in Great Britain crashed by up to 95% as a result of a myxomatosis epizootic in the 1950s (Sumption and Flowerdew 1985), the diet composition and population dynamics of other mustelid carnivorans, such as stoats Mustela erminea and weasels Mustela nivalis, were affected (McDonald et al. 2000). Rabbit populations recovered to their pre-myxomatosis levels by the 1990s (Aebischer et al. 2011; Aebischer 2019), but since then, rabbit numbers have declined across Britain (England − 44%; Scotland − 82%; and Wales − 48%; Harris et al. 2018), possibly as the result of rabbit haemorrhagic disease (RHD), which has devastated rabbit populations across mainland Europe (Lees and Bell 2008). Analysis of changes in rabbit records between 2011 and 2015 reveals spatial variation in rabbit declines, with the greatest reductions in central and southern England, along the Scottish borders and in north-east Scotland (Massimino et al. 2018).
In Spain, generalist carnivorans (such as red fox Vulpes vulpes, badger Meles meles and genet Genetta genetta) reduced their consumption of rabbits in response to declines in rabbit populations following RHD outbreaks (Ferreras et al. 2011). In contrast, whilst rabbit consumption by Iberian lynx Lynx pardinus, which are near-obligate predators of rabbits, also reduced, lynx continued to preferentially select rabbits in spite of their reduced availability (Ferreras et al. 2011). Given that polecats in Britain are known to eat rodents and amphibians as well as rabbits (Blandford 1986; Birks and Kitchener 1999), it is possible that reductions in rabbit populations would lead to polecats diversifying their diet.
Rodents are thought to be the major route by which polecats are exposed to second-generation anticoagulant rodenticides (SGARs) in Britain (Shore et al. 2003). Secondary exposure of polecats to SGARs increased 1.7-fold between 1993 and 2016, and the most recent study indicated that 79% of polecats had been exposed (Sainsbury et al. 2018). It may be that this increase has been a result of an increase in the proportion of rodents in polecat diet. Secondary exposure to SGARs may be lethal in sufficient concentration, or lead to a range of sub-lethal effects (Van den Brink et al. 2018). Increased rates of secondary exposure to SGARs have not prevented polecat expansion over the same time period (Sainsbury et al. 2019), but whether or not polecat abundance or the rate of population expansion have been affected by SGARs exposure is unknown (Sainsbury et al. 2018).
Between-sex dietary differences have been observed in some mustelids (McDonald 2002). Studies of polecat diet in Britain have previously found that female polecats tend to eat fewer rabbits and more birds than male polecats, though these differences were not statistically significant (Blandford 1986; Birks and Kitchener 1999). It is possible that as rabbit abundance has declined, increased competition for available rabbits may have led to more pronounced dietary differences between male and female polecat diets.
To explore dietary variation and niche breadth in polecats during the process of polecat population recovery and rabbit population variation, we analysed gut contents from polecats collected from 2012 to 2016 and compared our findings with earlier analyses of polecat diet in Britain in the 1960s (Walton 1968), 1980s (Blandford 1986) and 1990s (Birks and Kitchener 1999). We hypothesised that (i) rabbits will be reduced in importance in polecat diet compared with the 1990s in response to reduced rabbit abundance; (ii) rodent prey will have increased as a proportion of diet over the same period; (iii) polecat dietary niche breadth will have fluctuated over time, in line with known long-term variations in rabbit abundance and (iv) rabbit consumption will have differed between the sexes.
Materials and methods
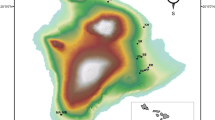
Polecat carcasses, predominantly of animals killed on the road, were collected across Great Britain during the Vincent Wildlife Trust’s national polecat survey in 2012–2016 (Croose 2016). Date of collection and location were recorded. Animals were stored frozen until necropsy examination, which was carried out at National Museums Scotland. Stomach contents were collected from 99 polecats (Fig. 1) and refrozen prior to dietary analysis. Stomach contents were soaked in biological detergent for 24 h, rinsed through a 53-μm sieve, then stored in 70% ethanol. Identifiable macroscopic animal remains (undigested body parts, fragments of bone, feathers, fur, individual hair and insect remains) were separated from unidentifiable tissues. Ten samples were selected at random and were analysed for earthworm (Lumbricidae) chaetae and other microscopic remains (after Reynolds and Aebischer 1991). No identifiable microscopic remains were found. As there is no evidence from previous dietary studies to suggest that polecats eat earthworms (e.g. Birks and Kitchener 1999), and since variation in microscopic remains do not relate to the primary processes of interest in this study, we considered only macroscopic remains in the remaining 90% of gut samples. Plant debris was considered to have been ingested when catching prey (Walton 1968) and was not included in diet composition quantification.
Fur remains were identified using guard hair cuticle patterns after Teerink (1991). All loose hairs were collected and analysed. Cuticle patterns were examined under a microscope at ×40 magnification. Mammal remains were identified to species level, except for rabbits and brown hares Lepus europaeus, which were not separated and were classified as lagomorphs. Most bones were fragmented and unidentifiable, but those that were intact, together with teeth, were identified as insectivore, rodent, larger mammal or amphibian to species level using personal collections and appropriate keys (e.g. Thomas 2008; Inns 2011). Bird remains were identified to order using Day (1966). Amphibians were determined by skin texture and, where possible, by webbing on feet (Inns 2011). Fishes were identified by their bones and scales but were not identified to species, as fish were a rare item and not of primary interest.
Two methods were used to assess accuracy when identifying guard hair cuticle pattern. First, 10% of samples were randomly selected for a second blind analysis by the same analyst; the correspondence in the results was 100%. A third analysis, again blind, was carried out by a second researcher, this time on 10% of samples that contained hard parts and 20% of the samples that relied on hair identification. There was a 100% match between analysts for samples containing hard parts, an 86% correspondence for hair samples and a calculated Cohen’s kappa test of interrater agreement of 0.7, which is “substantial” according to Landis and Koch (1977). The level of overlap in identification indicated that identification of guard hair was sufficiently robust for inclusion in our data analysis.
All analyses were carried out in R (R Core Team 2011). Diet was summarised as the percentage frequency of occurrence (% FO), calculated as the number of each type of dietary item as a percentage of the total number of identifiable prey items (e.g. Lodé 1994; McDonald et al. 2000). As our objective was to evaluate changes in polecat diet over time rather than assess differences in energetic requirements, frequency of occurrence was chosen as the best method for comparison as it was consistent with historical studies. Whilst frequency of occurrence may overestimate the importance of smaller food items in a carnivore’s diet, and caution should be used applying it in isolation to understand the effect of predators on prey populations, it is still an appropriate method for exploring a carnivore’s ecology (Klare et al. 2011). Polecats, in line with other small carnivores (e.g. McDonald et al. 2000), usually only have one prey item per stomach (e.g. Weber 1989a; Birks and Kitchener 1999). This means that the difference between frequency of occurrence calculated using total prey items or that using the number of stomachs is negligible (in this study, of the 99 polecat stomachs investigated and 79 that had identifiable contents, only three individual stomachs contained more than one item). As a result and for simplicity, we calculated frequency of occurrence per food item, expressed as a percentage of the number of occurrences of one food item of the total number of occurrences of all food items, to indicate the relative importance in diet (Klare et al. 2011). The frequency of occurrence matrix was replicated randomly 1000 times (bootstrapped with replacement 1000 times) to generate 95% confidence intervals following Reynolds and Aebischer (1991). Differences in FO of prey groups were compared using a chi-squared test.
Levins’ (1968) index of niche breadth was calculated following the formula:
where pi is the proportion of records for each species in each group. The proportion of prey records for each group was bootstrapped with replacement 1000 times to generate 95% confidence intervals for Levins’ index (Reynolds and Aebischer 1991).
To analyse variation in the occurrence of lagomorphs in polecat diet in more depth using the 2010s data, a binomial logistic regression model of presence/absence was fitted to sex, season (where spring is March to May, summer is June to August, autumn is September to November and winter is December to February) and region (north, south, east and west based on British government regions) as explanatory variables. Backwards stepwise model selection using the “drop1” function in R (with P = 0.05 used as the significance level to assess whether or not variables should be retained) was carried out to find the most parsimonious model.
Our results were compared with earlier polecat dietary studies by Walton (1968), Blandford (1986) and Birks and Kitchener (1999). Some of the data collected historically was from stomach contents (Walton 1968; Birks and Kitchener 1999) and some from scat analyses (Blandford 1986), which created a potential source of sampling bias. Scats may be considered similar to intestinal or rectal contents and therefore a separate meal to that found in the stomach (Day 1968). Comparisons between stomach and intestinal content of stoats (Mustela erminea: Day 1968; M. e. cicognanii: Aldous and Manweiler 1942) and common weasels Mustela nivalis (Day 1968) found no difference between stomach and intestinal content analysis in terms of dietary reconstruction, and therefore, it was considered acceptable to compare polecat diets between all of the historical studies.
To compare changes in frequency of occurrence of prey groups over time, binomial logistic regressions were run for the prey groups: all mammals, birds and amphibians for the 1960s–2010s datasets using decade as an explanatory variable. In addition, binomial logistic regressions were run for the prey groups: all mammals, lagomorphs, other mammals, birds and amphibians using the 1980s, 1990s, and 2010s datasets with decade and sex as explanatory variables. As backwards stepwise deletion (with P = 0.05 as the significance level used to assess whether or not variables should be retained in models) found that sex was not significant in any of the models, it was excluded from the analysis and only the results for the prey groups lagomorphs and other mammals are reported (as the models that included the 1960s datasets already include the other main prey groups). Models were not run for the prey groups fish and invertebrates due to small sample sizes.
Levins’ niche breadth was calculated using five categories (all mammals, birds, amphibians, fishes and invertebrates) for comparisons between 1960s, 1980s, 1990s and 2010s as the 1960s data did not distinguish mammal species. For the male and female calculations by decade between the 1980s and 2010s (the original 1960s data did not distinguish between the sexes), Levins’ niche breadth was calculated based on six categories (lagomorphs, other mammals, birds, amphibians, fishes and invertebrates) as data from the 1960s did make this distinction. Differences in niche breadth over time (1960s–2010s) and between the sexes (1980s–2010s) were compared.
Results
Of the 99 stomachs that contained some remains (65 male, 32 female, 2 sex unknown), 14 contained only liquid and six contained unidentifiable remains, such as undigested flesh. This left 79 stomachs containing identifiable prey items (54 male, 24 female, 1 unknown). Sixteen stomachs contained polecat hair, but this was excluded from the dietary analysis as it was assumed to be the result of grooming (Rysava-Novakova and Koubek 2009). Ten stomachs contained plant matter.
The observed frequencies of occurrence of the prey groups differed significantly from even (χ24 = 114.8, P < 0.001). Mammals were the most frequently identified prey group (78% FO), and lagomorphs comprised 66% FO of prey items (Table 1). Eight of the 54 lagomorph samples (15% FO) were neonates or juveniles, identified on the basis of intact ears, tails or feet. “Other mammals” comprised 12% FO of dietary items. Amphibians were the second most frequently identified prey group (10% FO) and mostly comprised frogs. Birds, fishes and invertebrates comprised approximately 7%, 4% and 1% FO of all items, respectively. Only one instance of carrion was found (identified based on the presence of maggots with the flesh), which was classified as lagomorph.
The % FO of all prey items in male and female polecat stomachs was similar across the board, and there was no difference in niche breadth between male (Levins’ index = 2.2, 95% CI 1.6–3.0) and female polecats (2.2, 95% CI 1.4–3.2).
The model analysing the factors influencing polecat diet in the 2010s found that season was the only factor that significantly affected the occurrence of lagomorphs (Fig. 2). Sex and region were removed from the model. The FO of lagomorphs was lowest in the autumn (Table 2) and significantly greater in spring (coefficient estimate 2.19, standard error 0.69, z = 3.18, P = 0.001) and summer (coefficient = 0.60, SE = 0.70, z = 0.85, P = 0.396) than in the autumn, but FO in winter (coefficient = 2.19, SE = 0.69, z = 3.18, P = 0.001) was not statistically significantly greater than in the autumn. Niche breadth was highest in autumn and lowest in the spring (Levins’ index, 95% CI spring 1.1, 1.0–1.3; summer 1.9, 1.2–3.1; autumn 4.1, 2.5–5.0; winter 2.6, 1.4–4.3; Fig. 2).
Percentage frequency of occurrence of five categories of prey identified in polecat stomachs from animals collected between 2012 and 2016, presented by season and as a percentage of prey items collected. Total n polecats = 79. Total n prey items = 82. Seasons are spring: March–May (n items identified = 24), summer: June–August (n = 20), autumn: September–November (n = 24) and winter: December–February (n = 13). One individual not shown in the graph but included in the main analyses did not have the date recorded. One polecat in summer and two polecats in autumn had two identifiable items in their stomachs
Analysis of changes in polecat diets over time indicated that the occurrence of all mammals more than doubled between the 1960s (35%) and the 1980s (74%), after which the occurrence of mammals stabilised as a proportion of diet (Table 3). The change in FO of mammals was statistically significant between the 1960s and all other decades, but the FO of mammals was the same between the 1980s and 1990s–2010s and the 1990s and 2010s (Table 4). Birds decreased significantly as a proportion of diet between the 1980s and 1990s and the 1980s and 2010s, but was similar in the 2010s compared with the 1990s (Table 4). Amphibians decreased significantly in frequency between the 1960s and 1980s but increased again in the 2010s (Table 3). Amphibian frequency was significantly lower in all other decades compared with the 1960s, but there was no significant difference in amphibians between any other decades (Table 4). Invertebrates decreased as a proportion of diet after the 1960s and were found only at low levels since then (1.1–2.0% of diet; Table 3). Lagomorph occurrence increased significantly between the 1980s (25%) and 1990s (69%) but did not differ between samples collected in the 1990s and the 2010s (66%; Tables 3 and 4). Other mammals increased in polecat diet between the 1980s and 1990s and 2010s. There was no significant difference between males and female polecats in FO of all mammals (including lagomorphs and other mammals), birds or amphibians. Polecat dietary niche breadth was greatest in the 1960s and lowest in the 1990s and 2010s (Table 3).
Discussion
Polecat diet composition in Great Britain was dominated by lagomorphs in all the samples analysed from the 1980s to 2010s, although there was seasonal variation in the 2010s, which reflects the opportunistic foraging strategy of this species. Contrary to our expectations, there was no evidence of a reduction in rabbit consumption by polecats since the 1990s, despite declines in rabbit records over this period (Harris et al. 2018). Instead, we found an increase in the frequency of occurrence of mammals in polecat diet since the 1960s and an increase in lagomorphs between the 1980s and 1990s, which is consistent with similar variation in the importance of lagomorphs in the diet of stoats over the same time period (McDonald et al. 2000). We also found that lagomorphs were equally important as prey for both male and female polecats in the 1990s and 2010s (Table 1) and so there was no evidence of resource partitioning between the sexes, consistent with McDonald (2002). We found evidence of polecat predation on young rabbits, which was also found in the 1990s study (Kitchener, unpublished data).
There are several possible explanations for this lack of dietary shift in response to declining resources. One is that the more recent rabbit declines have been uneven across the landscape (Massimino et al. 2018), unlike the 1950s myxomatosis epizootic (Sumption and Flowerdew 1985), and so, polecats have still been able to find and take rabbits as their major prey item. When rabbit numbers were still low in Wales in the 1980s (Aebischer et al. 2011) and polecats were predominantly found only in that country (Sainsbury et al. 2019), lagomorphs comprised a lower proportion of polecat diet than in the 2010s and niche breadth was correspondingly greater than in the 1990s or 2010s (Table 3; Blandford 1986). There was also some evidence of differences in resource use between males and females in the 1980s (lower lagomorph consumption by females) when rabbits were limited in availability (Table 3; Blandford 1986); this resource partitioning was not evident in the 2010s.
The continued high prevalence of rabbits in polecat diets means that other prey items, notably rodents, were consumed less frequently than might have been expected. The relatively high occurrence of amphibians is in line with polecat diet studies in Switzerland (Weber 1989a), Poland (Jędrzejewski et al. 1993; Jędrzejewska and Jędrzejewska 1998), France (Lodé 2000), and Denmark (Hammershøj et al. 2004). Whilst some dietary studies have correlated amphibian consumption to periods of abundance (Lodé 2000), others have found that polecats eat them preferentially (Weber 1989b). Data on nationwide trends in amphibians in Britain are limited, but available evidence that common toad Bufo bufo have been declining over the last 40 years (Petrovan and Schmidt 2016). Unlike in France, where amphibians are more commonly eaten in spring when amphibians are more active at the beginning of their breeding season (Lodé 2000), amphibians were most commonly caught in autumn in this study. The skin of the common toad was consumed intact in spite of its dermal secretions (Sidorovich and Pikulik 1997).
Given recent increases in the rates of exposure of polecats to anticoagulant rodenticides (Sainsbury et al. 2018), it is perhaps surprising that we did not observe an increase in the proportion of rodents, especially brown rats, consumed by polecats over this same time period. Furthermore, our data indicate that the current high proportion of polecats exposed to SGARs is associated with an intake of rodents that comprises less than 10% of total diet. This suggests that even relatively low rates of rodent consumption can result in high rates of secondary exposure. This means that our ability to minimise exposure of polecats to SGARs may be limited, as rodents comprise only a small component of their diet.
The recent occurrence of birds in the diet of polecats was consistent with that observed in previous decades. However, the importance of bird remains is often underestimated in analysing stomach contents (Reynolds and Aebischer 1991). Furthermore, polecats eat eggs, but we did not detect any evidence of this, possibly because polecats tend to break them open and lick out the contents (Weber 1989a). In the 1980s, Blandford (1986) found that Galliformes comprised 5% FO of polecat diet. This study was carried out before polecats had expanded beyond the Welsh border counties, an area where game management is less widespread (Tapper 1992). Birks and Kitchener (1999), who collected roadkill carcasses from across Wales and the English Midlands, did not identify any Galliformes in polecat stomachs, and our results are broadly consistent with this. Polecats spend time on game estates in Britain (Packer and Birks 1999), and in other countries, polecats are known to consume wounded or dead gamebirds (Rysava-Novakova and Koubek 2009). Given this and the difficulties associated with detecting birds in stomach contents, it is possible that birds might be eaten more frequently than our results suggest.
We have highlighted long-term increases in the proportion of lagomorphs in polecat diet in Britain during a period of polecat population recovery. When rabbits were almost extirpated from Britain in the 1950s, polecat diet was significantly more diverse. Polecat niche breadth has declined as rabbit populations have recovered. Our results, which show how niche breadth and diet composition has varied over time, demonstrate the importance of long-term studies for determining whether species are generalist or specialists. Overall, rabbits are an important prey item for polecats in Great Britain. Although rabbit populations are once again in decline, there is no evidence of a concomitant reduction of consumption in polecat diet, but it is possible that such effects may only be apparent from studies conducted at a finer spatial scale. Finally, our study shows that rodents do not have to constitute a high proportion of polecat diet, to lead to frequent secondary exposure to anticoagulant rodenticides.
References
Aebischer NJ (2019) Fifty-year trends in UK hunting bags of birds and mammals, and calibrated estimation of national bag size, using GWCT’s National Gamebag Census. Eur J Wildl Res 65:64
Aebischer NJ, Davey PD, Kingdon NG (2011) National Gamebag Census: mammal trends to 2009. Game & Wildlife Conservation Trust, Fordingbridge
Aldous ST, Manweiler J (1942) The winter food habits of the short-tailed weasel in Northern Minnesota. J Mammal 23:250–255
Amundsen PA (1995) Feeding strategy of Arctic charr (Salvelinus alpinus): general opportunist, but individual specialist. Nord J Freshw Res 71:150–156
Baghli A, Engel E, Verhagen R (2002) Feeding habits and trophic niche overlap of two sympatric Mustelidae, the polecat Mustela putorius and the beech marten Martes foina. Z Jagdwiss 48:217–225
Birks JDS (1998) Secondary rodenticide poisoning risk arising from winter farmyard use by the European polecat Mustela putorius. Biol Conserv 85:233–240
Birks JDS, Kitchener AC (1999) The distribution and status of the polecat Mustela putorius in Britain in the late 1990s. Vincent Wildlife Trust, London
Blandford PRS (1986) Behavioural ecology of the polecat Mustela putorius in Wales. PhD Thesis, University of Exeter, Exeter, UK
Blandford PRS (1987) Biology of the polecat Mustela putorius: a literature review. Mammal Rev 17:155–198
Croose E (2016) The distribution and status of the polecat (Mustela putorius) in Britain 2014–2015. Vincent Wildlife Trust, Ledbury
Day MG (1966) Identification of hair and feather remains in the gut and faeces of stoats and weasels. J Zool 148:201–217
Day MG (1968) Food habits of British stoats (Mustela erminea) and weasels (Mustela nivalis). J Zool 155:485–497
Erlinge S (1986) Specialists and generalists among the mustelids. Lutra 29:5–11
Ferreras P, Travaini A, Zapata SC, Delibes M (2011) Short-term responses of mammalian carnivores to a sudden collapse of rabbits in Mediterranean Spain. Basic Appl Ecol 12:116–124
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Hammershøj E, Thomsen EA, Madsen B (2004) Diet of free-ranging American mink and European polecat in Denmark. Acta Theriol 49:337–347
Harris SJ, Massimino D, Gillings S, Eaton MA, Noble DG, Balmer DE, Procter D, Pearce-Higgins JW, Woodcock P (2018) The Breeding Bird Survey 2017. BTO research report number 706. Peterborough
Inns H (2011) Britain’s reptiles and amphibians: a guide to the reptiles and amphibians of Great Britain, Ireland and the Channel Islands. WILDGuides, UK
Jędrzejewska B, Jędrzejewski W (1998) Predation in vertebrate communities: the Bialowieza Primeval Forest as a case study. Springer, Berlin
Jędrzejewski W, Jędrzejewska B, Brzezinski M (1993) Winter habitat selection and feeding habits of polecats (Mustela putorius) in the Bialowieza National Park, Poland. Z Säugetierkd 58:75–83
Klare U, Kamler JF, Macdonald DW (2011) A comparison and critique of different scat-analysis methods for determining carnivore diet. Mammal Rev 41:294–312
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Langley PJW, Yalden DW (1977) The decline of the rarer carnivores in Great Britain during the nineteenth century. Mammal Rev 7:95–116
Lee CE, Gelembiuk GW (2008) Evolutionary origins of invasive populations. Evol Appl 1:427–448
Lees AC, Bell DJ (2008) A conservation paradox for the 21st century: the European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal Rev 38:304–320
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton
Lodé T (1994) Environmental factors influencing habitat exploitation by the polecat Mustela putorius in western France. J Zool 234:75–88
Lodé T (1995) Activity pattern of polecats Mustela putorius L. in relation to food habits and prey activity. Ethology 100:295–308
Lodé T (1997) Trophic status and feeding habits of the European polecat Mustela putorius L. 1758. Mammal Rev 27:177–184
Lodé T (2000) Functional response and area-restricted search in a predator: seasonal exploitation of anurans by the European polecat, Mustela putorius. Austral Ecol 25:223–231
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Malecha AW, Antczak M (2013) Diet of the European polecat Mustela putorius in an agricultural area in Poland. Folia Zool 62:48–54
Massimino D, Harris SJ, Gillings S (2018) Evaluating spatiotemporal trends in terrestrial mammal abundance using data collected during bird surveys. Biol Conserv 226:153–167
McDonald RA (2002) Resource partitioning among British and Irish mustelids. J Anim Ecol 71:185–200
McDonald RA, Webbon C, Harris S (2000) The diet of stoats (Mustela erminea) and weasels (Mustela nivalis) in Great Britain. J Zool 252:363–371
Packer JJ, Birks JD (1999) An assessment of British farmers’ and gamekeepers’ experiences, attitudes and practices in relation to the European polecat Mustela putorius. Mammal Rev 29:75–92
Perry G, Pianka ER (1997) Animal foraging: past, present and future. Trends Ecol Evol 12:360–364
Petrovan SO, Schmidt BR (2016) Volunteer conservation action data reveals large-scale and long-term negative population trends of a widespread amphibian, the common toad (Bufo bufo). PLoS One 11:e0161943
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
R Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reynolds JC, Aebischer NJ (1991) Comparison and quantification of carnivore diet by faecal analysis: a critique, with recommendations, based on a study of the fox Vulpes vulpes. Mammal Rev 21:97–122
Rosecchi E, Thomas F, Crivelli AJ (2001) Can life-history traits predict the fate of introduced species? A case study on two cyprinid fish in southern France. Freshw Biol 46:845–853
Rysava-Novakova M, Koubek P (2009) Feeding habits of two sympatric mustelid species, European polecat Mustela putorius and stone marten Martes foina, in the Czech Republic. Folia Zool 58:66–75
Sainsbury KA, Shore RF, Schofield H, Croose E, Pereira MG, Sleep D, Kitchener AC, Hantke G, McDonald RA (2018) Long-term increase in secondary exposure to anticoagulant rodenticides in European polecats Mustela putorius in Great Britain. Environ Pollut 236:689–698
Sainsbury KA, Shore RF, Schofield H, Croose E, Campbell RD, McDonald RA (2019) Recent history, current status, conservation and management of native mammalian carnivore species in Great Britain. Mammal Rev 49:171–188
Santos MJ, Matos HM, Baltazar C, Grilo C, Santos-Reis M (2009) Is polecat (Mustela putorius) diet affected by “mediterraneity”? Z Säugetierkd 74:448–455
Shore RF, Birks JDS, Afsar A, Wienburg CL, Kitchener AC (2003) Spatial and temporal analysis of second-generation anticoagulant rodenticide residues in polecats (Mustela putorius) from throughout their range in Britain, 1992–1999. Environ Pollut 122:183–193
Sidorovich VE, Pikulik MM (1997) Toads Bufo spp. in the diets of mustelid predators in Belarus. Acta Theriol 42:105–108
Sumption KJ, Flowerdew JR (1985) The ecological effects of the decline in rabbits (Oryctolagus cuniculus L.) due to myxomatosis. Mammal Rev 15:151–186
Teerink BJ (1991) Atlas and identification key hair of West-European mammals. Cambridge University Press, Cambridge
Thomas L (2008) Guide to British owls and owl pellets. Field studies council occasional publication number 123. Field studies council, UK
Van den Brink NW, Elliott JE, Shoe RF, Rattner BA (eds) (2018) Anticoagulant rodenticides and wildlife. Springer international publishing AG, Cham
Walton KC (1968) Studies on the biology of the polecat, Putorius Putorius (L). MSc dissertation, Durham University, Durham, UK
Weber D (1989a) The diet of polecats (Mustela putorius L.) in Switzerland. Z Säugetierkd 54:157–171
Weber D (1989b) Foraging in polecats (Mustela putorius L.) of Switzerland: the case of a specialist anuran predator. Z Säugetierkd 54:377–392
Zhou YB, Newman C, Xu WT, Buesching CD, Zalewski A, Kaneko Y, Macdonald DW, Xie ZQ (2011) Biogeographical variation in the diet of Holarctic martens (genus Martes, Mammalia: Carnivora: Mustelidae): adaptive foraging in generalists. J Biogeogr 38:137–147
Acknowledgements
We thank all polecat carcass contributors, M. Bruce and M. Chappell for processing stomach contents and initial analysis and L. Walker and E. Potter for managing carcass collection as part of the Predatory Birds Monitoring Scheme. ACK is grateful to the Negaunee Foundation for its continuing generous support of a preparator at National Museums Scotland. KS was supported by a PhD studentship funded by Vincent Wildlife Trust and the College of Life and Environmental Sciences, University of Exeter.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Andrzej Zalewski
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sainsbury, K.A., Shore, R.F., Schofield, H. et al. Diets of European polecat Mustela putorius in Great Britain during fifty years of population recovery. Mamm Res 65, 181–190 (2020). https://doi.org/10.1007/s13364-020-00484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-020-00484-0