Abstract
Characterization of acidic peptides and proteins is greatly hindered due to lack of suitable analytical techniques. Here we present the implementation of 213 nm ultraviolet photodissociation (UVPD) in high-resolution quadrupole-Orbitrap mass spectrometer in negative polarity for peptide anions. Radical-driven backbone fragmentation provides 22 distinctive fragment ion types, achieving the complete sequence coverage for all reported peptides. Hydrogen-deficient radical anion not only promotes the cleavage of Cα–C bond but also stimulates the breaking of N–Cα and C–N bonds. Radical-directed loss of small molecules and specific side chain of amino acids are detected in these experiments. Radical containing side chain of amino acids (Tyr, Ser, Thr, and Asp) may possibly support the N–Cα backbone fragmentation. Proline comprising peptides exhibit the unusual fragment ions similar to reported earlier. Interestingly, basic amino acids such as Arg and Lys also stimulated the formation of abundant b and y ions of the related peptide anions. Loss of hydrogen atom from the charge-reduced radical anion and fragment ions are rationalized by time-dependent density functional theory (TDDFT) calculation, locating the potential energy surface (PES) of ππ* and repulsive πσ* excited states of a model amide system.

ᅟ
Similar content being viewed by others
Introduction
Alternative to collision [1–3] and electron [4, 5] based techniques, photon-based methods have emerged as new powerful approaches for characterizing peptides, polysaccharides and proteins [6–12]. Among them, ultraviolet photodissociation (UVPD) leads to intense fragmentation patterns. In this method, protein and peptide cations predominately dissociate to a/x ions and less frequently to c/z and b/y ions. Different wavelengths such as 157, 193, 220, and 280 nm have been implemented in UVPD. Above and at 280 nm, specific fragmentation has been reported following excitation of aromatic residues in peptides or proteins [13]. The number of fragment ions increases as the wavelength decreases from 280 to 213 nm [13, 14].
Another efficient and popular wavelength 193 nm has been implemented in hybrid linear ion trap-Orbitrap mass spectrometer for characterizing different peptide and proteins in positive polarity. Wide-ranging fragmentation yields a/x, b/y, c/z, y-1, v, w, and d ions and thus provides nearly complete sequence coverage. Whole protein characterization has been achieved by this technique implementing direct infusion and/or chromatographic time scale [15, 16]. Along with common fragment ions, Madsen et al. also observed some uncommon fragment ions such as a + 2, c – 1 and z + 1 [17]. This study disclosed that fragmentation patterns varied with the protonation state of the peptide. When protonation takes place at N-terminus, cleavage of Cα–C bond occurred; however, N–Cα cleavage is favored with C-terminus protonation.
Thompson et al. employed vacuum photodissociation at 157 nm on singly protonated peptide ions to elucidate the unusual backbone cleavage [18]. Cui et al. further revealed that basic residues in the C-terminal yields to x, v, and w fragment ions, whereas N-terminal produces a and d fragments ions [19]. Moreover, a + 1 and x + 1 radical ions are identified from the charge localized N- and C-terminals, respectively. Secondary radical elimination of hydrogen atom are detected from a + 1 and x + 1 ions to produce a and x ions, respectively. Satellite ions such as d, v, and w are formed due to part of side chain elimination; b, c, and z fragment ions are also noticed but are less frequent than a and x ions. Hydrogen/deuterium exchange experiments further confirmed that both backbone amide and side-chain β-carbon hydrogens can undergo elimination to yield a and x ions [20]. Implementing time-resolved photodissociation at 157 nm revealed some unusual but stable x + 2 fragment ion compared with less common a + 2 ion [21]. They proposed that addition of one hydrogen to x + 1 and a + 1 radical ions can yield x + 2 and a + 2 ions. Migration and transfer of hydrogen atom to radical ions have also been witnessed in ECD studies [22, 23].
However, most of these experiments were conducted on peptide and protein cations and very few were directed on negative polarity. It is assumed that around 50% of naturally occurring peptides are acidic and prone to yield negative ions. Kjeldsen et al. reported Cα–C backbone fragmentation by EDD (electron detachment dissociation) for peptide and observed more C-terminal species (x ions) than N-terminal fragments (a• ions) [24]. Comparison of negative electron transfer dissociation (NETD) and UVPD for peptide anion disclosed that NETD usually produce simple set of a/x ions [25]. In NETD, along with a/x ions various neutral losses are observed from entire or partial side-chain cleavage of amino acids [26]. Activated ion negative electron transfer dissociation (AI-NETD) of doubly charged peptide ions also generates some hydrogen loss from a and x fragment ions [27].
Some previous electron photo-detachment dissociation (EPD) studies were performed with UV lasers on peptides and small proteins in negative polarity [28, 29]. Antoine et al. investigated the electron photo-detachment dissociation of peptides using 262 nm with a linear ion trap [30]. Formation of [M – 2H]-• radical anion from the precursor ion was documented in this experiment; a/x and c/z fragment ions were observed [28]. Comparative studies between EDD and EPD revealed significantly different fragment ion distributions in which EPD fragment ions are typically produced from tryptophan and histidine resides whereas in EDD backbone dissociation are favored [28]. However, EDD on small proteins including ubiquitin and melittin suggests that basic resides may promote the formation of a/x fragment ions [31].
Radical-containing peptides promote characteristic fragmentation pattern in mass spectrometry [32, 33]. Radical peptides are classified into two categories: hydrogen-deficient and hydrogen-rich radicals [34]. The former type is typically formed in UVPD, EDD, and NETD routes, whereas the latter is generated from ECD/ETD [8, 24, 35–37]. Recently, formation of hydrogen-deficient species from the hydrogen-rich radical cation in ECD received great attention because of extensive fragmentation and widespread side-chain loss [33, 38]. Radical migration in hydrogen-deficient peptide radical promotes extensive neutral loss and allows remote backbone dissociation [33, 39].
Here, we present the implementation of 213 nm UVPD in a Thermo Scientific Q Exactive hybrid quadrupole-Orbitrap mass spectrometer in negative polarity for peptide anions. We observed distinctive Cα–C, N–Cα, and C–N backbone fragmentations from the hydrogen-deficient radical anions. Radical-driven extensive neutral loss is likewise evident in these experiments. Moreover, series of hydrogen-deficient and hydrogen-rich fragments are observed.
Material and Methods
Photodissociation Mass Spectrometry
All experiments were performed on a hybrid quadrupole-Orbitrap Q-Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) equipped with a HESI ion source. Three small peptides YTIAALLSPYS, DYKDDDDK, and RGDSPASSKP were used without any further purification. Peptide samples were prepared at 1 μM concentration in 50/49/1 (v/v/v) acetonitrile/water/ammonium hydroxide and directly infused to MS at a flow rate of 5 μL/min. All spectra were acquired using a mass range of 100–1500 m/z and resolving power of 140,000 at m/z 400. The automatic gain control (AGC) target for MS/MS was set to 1 × 106 and the maximum injection time was set at 250 ms. The isolation width was 2 Th. When required, the identification of fragment ions was confirmed by fragmentation of a single isotope (selection width 0.4 Th). The high collision dissociation (HCD) collision energy was set to the minimum 2 eV in order to avoid collisions and provide photofragmentation spectra free of CID contamination. Different HCD trapping times including 100, 500, 1000, and 2000 ms (2, 10, 20, 40 laser shots, respectively) were considered. All experiments were performed on five microscans mode with averaging 200 scans.
For UVPD experiments, BrillantB Nd:YAG (Quantel, Les Ulis, France) laser was employed. Details of the setup are given elsewhere [14]. In brief, the 5th harmonic (λ = 213 nm) with a repetition rate of 20 Hz was used. The hybrid quadrupole-Orbitrap Q-Exactive mass spectrometer was modified to permit the laser irradiation of peptide ions. The laser beam passes through lenses, diaphragms, and then is introduced in the HCD cell using two dichroic mirrors. A UV grade fused-silica window was fitted on the back of the HCD cell to allow penetration of a laser beam. The laser beam energy irradiating the ions was ~1 mJ/pulse. The laser was slightly off-axis so as to avoid photofragmentation in the C-trap.
Manual analysis of UVPD data was performed with the aid of ChemCalc software [40]. Peak lists of three peptides were generated for all six major UVPD ion types (a, b, c, x, y, and z). Fragments mass tolerance was set to 20 ppm.
Computation
All calculations were conducted with the Gaussian 09 software package [41]. Optimization and subsequent vibrational frequency calculation on the model amide system CH3CONHCH3 were performed using density functional theory employing Becker’s (B3) [42] exchange functional combining Lee, Yang, and Parr’s (LYP) [43] correlation functional. Gaussian basis set 6-311+G (2d,p) was considered. Natural bond orbital (NBO) [44, 45] calculations were computed at the same level of theory. For calculating the excited state properties, time-dependent density functional theory (TDDFT) [46] was employed with the B3LYP/6-311+G(2d,p) level of theory in gas phase. For TDDFT calculation, 20 excited states were considered.
Result and Discussion
The Photodissociation of Peptide 1 (YTIAALLSPYS)
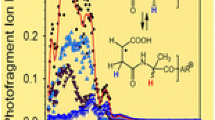
The photodissociation spectrum of the doubly deprotonated [M – 2H]2– (m/z 597.8057) of this peptide is presented in Figure 1a. Exact masses and assignments of fragment ions of this peptide are summarized in Table 1. Similar to previous studies, the characteristic [M – 2H]–• charge-reduced radical species is detected at m/z 1195.6094 Da. This radical species is typically generated from photo-induced electron detachment from the selected peptide precursor. Intense neutral losses are detected from this radical species (Table 2 and Figure 2). Similar neutral losses are also demonstrated in previous studies [30, 31, 47–49]. The CH3 radical (15.0242 Da) loss appears at m/z 1180.5852 from the side chain of Ala [50]. Neutral losses of CO (27.9947 Da) and CH3CH2 (28.9995 Da) are noticed at m/z 1167.6147 and 1166.6099 Da, respectively. Removal of CH3CH2 can be used to distinguish the side chain loss of Ile (28.9995 Da) or Leu (43.0542 Da) [51]. Loss of CH2O (30.0100 Da) and CH2OH (31.0178 Da) are also observed from the side chain of Ser. NETD study on Ser-containing peptide witnessed the loss of CH2O when Ser is not phosphorylated [26]. The peak at m/z 1151.5829 can be assigned to the loss of C2H4O (44.0265 Da) from Thr side chain [26, 50]. The sequential loss (61.9998 Da) of CO2 and H2O is also identified at m/z 1133.6099. Radical elimination of a C3H8ON from the Thr residue may lead to the fragment ion detected at m/z 1121.5759. Loss of tyrosylate groups from the side chain of Tyr (107.0472 and 106.0406 Da) is identified at m/z 1088.5622 and 1089.5688 Da, respectively. The phenoxy group of the tyrosylate produces an oxygen radical, which induces the cleavage of Cα–Cβ side chain of the tyrosine residue and promotes the formation of O=C6H4=CH2 (exact mass 106.0413 Da) ion [8, 50, 52]. Two relatively weak peaks at m/z 1139.5855 and 1123.5910 can be assigned for the side chain and related ion loss (56.0239 and 72.0184 Da) from Leu or Ile [26, 51–53]. Combined losses of tyrosylate and C2H4O from Tyr and Thr appear at m/z 1045.5419 and 1044.5346, respectively.
Photodissociation spectra of the doubly deprotonated [M – 2H]2– ion (m/z = 597.8057) of YTIAALLSPYS at 213 nm. The precursor ion is indicated by the * symbol and the neutral losses are indicated by ion masses. The green and blue lines represent the a, b, c and x, y, z ions, respectively. (a) Spectrum of 100–1200 m/z, (b) spectrum of 900–1100 m/z, and (c) spectrum of 600–900 m/z
Zooms of Figure 1a are shown in Figure 1b, c, and Supplementary Figure S1. Selected fragment ions from the single isotope selection of the doubly deprotonated [M – 2H]2– precursor ions are shown in Supplementary Figure S2. For peptide 1, a series of radical (an + 1)–. fragment ions is observed for n = 5, 6, 7, 8, and 9 These ions correspond to the elemental composition of an ions plus one hydrogen atom (explaining the +1 in the notation) and are radicals (dot in the notation). This nomenclature is in agreement with the one proposed recently by Chu et al [54] except that we do not include the hydrogen symbol (H) after the number of losses or gains. Homolytic cleavage between the Cα and the carbonyl C from the precursor ion induced the formation of these radical ions, as shown in Scheme 1. Classic (an)– fragment ions are detected for n = 8 and 9. These ions may mainly arise from the fragmentation of the doubly deprotonated [M – 2H]2– precursor ion. However, they can also be produced by secondary H elimination from the radical (an + 1)–. fragment ions [19]. Abundant a ions are favored by aromatic amino acids and in this case it is due to Tyr residue in N-terminal [28, 52]. An unusual fragment such as (a8 + 2)– is additionally identified at m/z 805.4815, which may be due to the presence of Pro residue [14, 17]. Detection of (a + 2)– is also reported by Madsen et al. in a high-throughput UVPD study in negative polarity for complex proteomic sample [55]. Two peaks at m/z 871.5031 and 856.4917 correspond to the loss of CH3CH2 (28.9995 Da from Ile) and C2H4O (44.0265 Da from Thr) from (a9)– ion. Radical (xn + 1)–. ions are also formed via homolytic cleavage of the Cα–carbonyl C bond, complementary to (an + 1)–. ions (Scheme 1). Series of radical (xn + 1)–. ions are noticed at n = 2, 5, 6, 7, 8, and 10, whereas (xn)– ions are detected at n = 6, 7, 8, and 9. Two unusual fragment types such as (xn + 2)– for n = 2, 8, and radical (xn – 1)–. for n = 7 and 9 appear for peptide 1, and (x2 + 2)– ion detected at m/z 295.0924 is close to Pro residue [14]. Kim and Reilly found xn + 2 fragment ions at 157 nm UVPD and concluded that some x + 1 radical ions may take one hydrogen to form these new ions [21]. (xn + 2)– ions are also detected at 193 nm UVPD [55]. The proposed fragmentation pathway for the formation of (x2 + 2)– ion is presented in Scheme 2.The formation of two (xn – 1)–. ions are likewise attributable to the radical elimination of hydrogen atom from the corresponding xn ions. Shaw et al. also observed some (xn – 1)–. ions in activated ion negative electron transfer dissociation [27]. Moreover, classic fragmentation of the Cα–C bond with proton transfers from the charge-reduced [M – 2H]–. radical species also yields to the formation of (xn – 1)–. ions. Indeed, these ions will contain the initial radical site and the negative charge. Fragmentation is then observed after electron photo-detachment.
Series of (yn)– ions are detected at n = 2, 3, 6, 7, and 8. Radical (yn – 1)–. ions are also observed at the positions n = 6, 7, 8, and 10. These ions arise from the homolytic cleavage of the C–N bond from the precursor ion (Scheme 3). However, complementary (bn + 1)–. radical ions are not detected. Fragmentation of the C–N bond from the charge-reduced [M – 2H]–. radical species may also leads to the formation of the (yn – 1)–. ions, if the charge and the radical site after electron loss are located on the C-terminal side. As a general statement, the abundance of fragment ions results from both direct fragmentation of the precursor ions and fragmentation of the charge-reduced radical ions obtained after electron loss (EPD). (yn – 1)–. radical ions could also be formed by H elimination from the (yn)– ions. Three new (yn – 2)– ions are detected for this peptide at n = 3, 8, and 9 positions and could be formed by H elimination from the (yn – 1)–. ions. The fragmentation of the C–N bond close to the Pro residue can also explain the formation of the (y3 – 2)– fragment ion [14]. Once again, these fragment ions could also arise from the homolytic cleavage of C–N bond fragmentation from the charge-reduced [M – 2H]–. radical species. One (b8 + 2)- fragment ion is detected at m/z 833.4757 for this peptide attributable to the presence of the Pro residue [14]. A neutral loss of 44.0264 Da corresponds to C2H4O of Thr observed at m/z 789.4493 from (b8 + 2)- (Figure 1a).
c/z Ions are less abundant for this peptide. Two (cn)- ions are detected at n = 7 and 9 positions. Moreover, two (cn – 1)–. ions at n = 9, 10 positions and (cn – 2)– ions at n = 7, 10 sites are observed. Radical (cn – 1)–. ions could be produced via the homolytic cleavage of the N–Cα bond from the precursor ion (Scheme 4). Hydrogen abstraction from c ions are also detected in ECD [22, 56, 57]. The formation of the (cn – 2)– ions could be explained by the radical induced fragmentation of the N–Cα bond from the charge-reduced [M – 2H]–. radical species after electron loss.
The Photodissociation of Peptide 2 (DYKDDDDK)
The photodissociation spectrum of the doubly deprotonated [M – 2H]2– (m/z 505.1906) of peptide DYKDDDDK is presented in Figure 3 and Supplementary Figure S3. Exact masses and assignments of fragment ions of this peptide are summarized in Table 3. Intense neutral losses are also evident from this peptide (Supplementary Table S1). Loss of H2O from the charge-reduced radical species [M – 2H]–• is detected at m/z 992.3709. Losses of one, two, and three CO2 are identified at m/z 966.3913, 922.4019, and 878.4116, respectively. Madsen et al. observed one and two CO2 loss at 193 nm UVPD of singly and multiply charged peptide anions [49]. Abundant CO2 loss was moreover demonstrated in electron detachment dissociation for peptide and protein [29, 30]. Elimination of several CO2 is a common feature related to aspartic and glutamic acid residues in NETD, Al-NETD, EDD, and UVPD [27, 30]. The UVPD spectrum showed losses of 27.9955 Da from [M – 2H]–• that can be attributed to CO, similar to peptide 1. Loss of CO from radical species is also found in an earlier ECD study [58]. The peaks at m/z 903.3321 and 904.3394 correspond to the losses of tyrosylate groups of Tyr (107.0491 and 106.0418 Da) from the [M – 2H]–•. Radical C3H6O2N (88.0371 Da) group elimination from the aspartic amino acid yields to the ion detected at m/z 922.3441. The ion observed at m/z 938.3961 can be assigned to the loss of C3H4O2 (71.9851 Da) from Asp residue [26]. Loss of Lys residue (100.0736 Da) is also detected at m/z 910.3076. Moreover, a loss of 71.0713 Da (C4H9N) observed for the ion at m/z 939.3099 is from the Lys residue [26]. A combined loss of CO2 and H 2 O appears at m/z 948.3803.
A complete series of (an)- fragment ion is observed for this peptide for n = 2–7; (an + 1)–. ions are detected for n = 4, 5, 6, and 7. These ions are formed via homolytic cleavage from the precursor ion (Scheme 1). Radical (an – 1)–. ions are detected for n = 3, 5, and 6. Fragmentation of the Cα–C bond from the charge-reduced radical species [M – 2H]–• is involved to produce these series. Secondary radical elimination of hydrogen atom from (an)– ions could also yield to the formation of these ions. A complete series of (xn)- fragment ions is detected at n = 2–7 similar to complementary (an)– ions. Two radical (xn + 1)–. ions (n = 3 and 6) are detected at m/z 402.1380 and 760.2859, respectively. Moreover, two (xn + 2)– ions (n = 2 and 6), which are formed by addition of one extra hydrogen atom to (xn + 1)– ions are detected. Additionally, (x7 – 1)– ion is observed at m/z 921.3364. Same fragmentation mechanisms are proposed for the formation of these ions than for the peptide 1 described previously. A distinctive peaks at m/z 886.3281 corresponds to the loss of two H2O molecules from (x7)–, respectively.
Two (bn)– fragment ions are observed at n =1 and 7 sites, whereas very abundant radical (bn – 1)–. ions are detected for n = 1, 3–7. These ions would come from the fragmentation of the C–N bond from the charge-reduced [M – 2H]–• radical species. Several (yn)– ions appear at n = 3–6 positions. Some (yn – 1)–. ions at n = 3, 6, 7 sites are also detected (formed via the mechanism proposed Scheme 3) as well as (y7 – 2)– ion. Specific radical induced fragmentation of the [M – 2H]–• radical species is then also observed, after electron loss, for this peptide.
Cleavage of N–Cα bonds produces series of c and z ions. Four (cn)– ions and (cn – 1)–. radical ions are noticed at n = 4–7 positions. These ions arise from the homolytic cleavage of the N–Cα bond from the precursor ion (Scheme 4). However, complementary (zn + 1)– radical ions are not detected. (zn)– ions are detected from 2, 3, 6, and 7 positions. Interestingly, complete series of radical (zn – 1)–. ions (n = 2–7) is observed for this peptide. Classic fragmentation of the N–Cα bond with proton transfers from the [M – 2H]–• radical species is proposed for the formation of these ions as well as the (cn – 1)–. series. Compared with the first peptide, abundance of c and z ions is noticeable for this peptide and may be due to the presence of five Asp residues. Removal of one H2O, one CO2, and combined CO2 and H2O from (z2)– ion are detected at m/z 225.0868 199.1074 and 181.0967, respectively. Previous studies also noticed the losses of H2O and CO2 from z ion when peptide contained Asp residues [56]. Combinations of backbone cleavages and neutral losses are listed in Supplementary Table S1.
The Photodissociation of Peptide 3 (RGDSPASSKP)
The photodissociation spectrum of the doubly deprotonated [M – 2H]2– (m/z 499.2393) of peptide RGDSPASSKP is presented in Figure 4 and Supplementary Figure S4. Exact masses and assignments of fragment ions of this peptide are summarized in Supplementary Table S2. Intense neutral losses are summarized in Supplementary Table S3. The loss of H2O from the charge-reduced radical species [M – 2H]–• (m/z 998.4767) is noticed at m/z 980.4673 (Figure 4a). There are three Ser residues in this peptides and loss of CH2O (30.0095 Da) at m/z 968.4672 can be attributed to the side chain of Ser. The loss of 60.0540 Da observed for the peak at m/z 938.4227 corresponds to the C2H6ON group of the Ser residue. Loss of CO2 (exact mass 43.9895 Da) from the carboxyl group located in C-terminal or side chain of aspartic acid appears at m/z 954.4872. Two distinctive peaks at m/z 899.3982 and 912.4072 correspond to the losses of 99.0785 and 86.0695 Da from the arginine side chain [26, 53]. Loss of 88.0498 Da, which is detected at m/z 910.4269, is related to the side chain of Asp [59].
Nearly complete series of (an)– fragment ions is observed for this peptide for n = 2–9, whereas (an – 1)–. ions are detected for n = 6 and 9. Radical (an + 1)–. ions are detected for n = 2–9 (Scheme 1). Addition of one hydrogen to (an + 1)–. radical ions (similar as shown in Scheme 2 for the xn + 1 ions), which yield (an + 2)– is also prevalent for n = 3–5, 7–9 positions; (an + 2)– ions are also observed for Proline containing peptides [14, 60] and explain the formation of (a4 + 2)–. and (a9 + 2)– ions. An almost complete series of (xn)– fragment ions is detected at n = 1–4, 6–9 similar to the complementary (an)– ions. Four (xn – 1)–. ions are observed for n = 1, 4, 7–9 sites. Moreover, (xn + 1)–. ions are detected for n = 1–4, 6, 7, and 9. Three (xn + 2)– ions (n = 2, 3, 6, and 7) are also formed via H addition on the (xn + 1)–. ions.
(bn)– and (yn)– fragments ions are predominant in this peptides, which may be due to the presence of basic Arg and Lys amino acids [61]; (bn)– ions are identified for n = 1–5, 8, and 9 positions only missing n = 6 and 7 related to Ala–Ser and Ser–Ser amide bonds; (bn + 1)–. ions are detected for n = 4, 5, and 9 (Scheme 3). Three (bn – 1)–. ions are observed at n = 3, 8, and 9. Representative (bn + 2)- ions appear at 2, 4, 9 positions in which two sites (4 and 9) are closed to the Pro residues; (b2 + 2)– ion could be explained by the H addition on the (bn + 1)–. ion. Complete sequence of (yn)– ions are found (n = 1-3, 5–9) whereas (yn – 1)–. ions are noticed for n = 2, 5–9. Distinctive (yn – 2)– ions are detected for n =1, 2, 6–9.
Homolytic cleavage and fragmentation, associated with proton transfers, of N–Cα bonds is also noticeable. Full sequence of (cn)– ions located for n = 1–3, 5–9, and (cn – 1)–. ions are noticed at n = 3, 6–9. Fragment (cn – 2)– ions are detected for n = 2, 3, 6–8. Similar to peptide 2, complete series of (zn)– ions (n =2–9) are generated from this peptide; (zn – 1)–. ions are also observed for n = 3, 7–9. Moreover, (zn + 1)–. ions are detected for n =2, 4, 6, 7, and 9 (Scheme 4).
Photo-Induced Hydrogen loss at 213 nm
A general trend is observed for those three peptides with series of backbone cleavages leading to ions deficient in hydrogen. All three peptides produce the distinctive doubly deprotonated [M – 2H]–• charge-reduced radical species upon irradiation of the monoisotopic precursor ion [M – 2H]2–, along with hydrogen loss from the charge-reduced radical species as shown in (Figure 5). Time-dependent density functional theory (TDDFT) calculation has been performed on a model amide system to elucidate the role of πσ* excited state in the photodissociation of peptide. The potential energy surface of the model amide system, π, π*, and σ* molecular orbitals are displayed in Figure 6. The lowest ππ*, πσ*, and electronic group state (S0) are shown with respect to the N–H stretching coordinate of the model amide. The ππ* excitation is observed for the amide system at 215 nm (5.75 eV), which relates with our UVPD experiment at 213 nm. The diffuse and polar character of σ* orbital is observed which is similar to the previous studies on pyrrole/indole system [62, 63]. The shallow barrier with respect to N–H stretch indicates the repulsive nature of this state [62]. For this amide system, the ππ* surface is above the πσ* surface, which may allow the fast internal crossing from the ππ* to the πσ* states and lead to H atom dissociation [63–65]. The ππ* excitation-induced amide hydrogen loss then provides a general route for the formation of hydrogen-deficient ions in 213 nm UVPD. Repetition of this mechanism with absorption of several photons can lead to fragments displaying multiple H-loss. Moreover, the ππ* excitation-induced amide hydrogen loss may yield a nitrogen-centered amide anion intermediate and stimulate the widespread backbone fragmentation. However, detailed theoretical calculations are sought to elicit the mechanism of radical-driven side-chain loss and backbone fragmentation at 213 nm photodissociation on peptide and protein anions. A similar mechanism can also arise on other bonds from aromatic cycles or COO chromophore groups.
Conclusion
The key features of these experiments can be summarized as follows: (1) Extensive sequence specific side-chain losses are observed for all three peptides. (2) Near complete series of classic backbone cleavages (a/x, b/y, c/z) are observed. (3) Unusual fragment ions including (x + 1)–., (x + 2)–, (x – 1)–., (y – 1)–., (y – 2)–, (z – 1)–., (z + 1)–., (z + 2)– , and (a – 1)–., (a + 1)–., (a + 2)–, (b – 1)–., (b + 1)–., (b + 2)–, (c – 1)–., (c – 2)– are consistently observed in these experiments and further confirmed by selecting single isotopic peak of the precursor ions. Some of these ions are coming from homolytic cleavages of the backbone from the precursor doubly charged ion. Classic fragmentation of backbone bonds concerted with proton transfers and homolytic cleavages are also observed for the charge-reduced [M – 2H]–. radical species after electron photo-detachment. Radical-induced specific fragment ions are then produced in these experiments of UVPD in the negative mode. Some of these ions may also result from secondary H eliminations. (4) Hydrogen-deficient ions may result from ππ* excitation-induced amide hydrogen loss. This ππ* excitation is reached upon absorption of a photon at 213 nm. The present study outlines the difficulty to interpret and systematically analyze the wealth of fragmentation produced by irradiation of peptide and protein anions at the onset of the amide bond absorption band, which may be different from VUV excitation.
References
Zhurov, K.O., Fornelli, L., Wodrich, M.D., Laskay, U.A., Tsybin, Y.O.: Principles of electron capture and transfer dissociation mass spectrometry applied to peptide and protein structure analysis. Chem. Soc. Rev. 42, 5014–5030 (2013)
McLuckey, S.A.: Principles of collisional activation in analytical mass spectrometry. J. Am. Soc. Mass Spectrom. 3, 599–614 (1992)
Wells, J. M., McLuckey, S. A.: Collision-induced dissociation (CID) of peptides and proteins. Methods Enzymol. 402, 148–185 (2005)
Zubarev, R.A., Kelleher, N.L., McLafferty, F.W.: Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 120, 3265–3266 (1998)
Syka, J.E.P., Coon, J.J., Schroeder, M.J., Shabanowitz, J., Hunt, D.F.: Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 101, 9528–9533 (2004)
Brodbelt, J.S.: Shedding light on the frontier of photodissociation. J. Am. Chem. Soc. 22, 197–206 (2011)
Brodbelt, J.S.: Photodissociation mass spectrometry: new tools for characterization of biological molecules. Chem. Soc. Rev. 43, 2757–2783 (2014)
Antoine, R., Lemoine, J., Dugourd, P.: Electron photodetachment dissociation for structural characterization of synthetic and biopolymer anions. Mass Spectrom. Rev. 33, 501–522 (2014)
Vasicek, L.A., Ledvina, A.R., Shaw, J., Griep-Raming, J., Westphall, M.S., Coon, J.J., Brodbelt, J.S.: Implementing Photodissociation in an Orbitrap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 22, 1105–1108 (2011)
Vasicek, L., Brodbelt, J.S.: Enhancement of ultraviolet photodissociation efficiencies through attachment of aromatic chromophores. Anal. Chem. 82, 9441–9446 (2010)
Smith, S.I., Brodbelt, J.S.: Characterization of oligodeoxynucleotides and modifications by 193 nm photodissociation and electron photodetachment dissociation. Anal. Chem. 82, 7218–7226 (2010)
Robinson, M.R., Madsen, J.A., Brodbelt, J.S.: 193 nm Ultraviolet photodissociation of imidazolinylated lys-N peptides for de novo sequencing. Anal. Chem. 84, 2433–2439 (2012)
Tabarin, T., Antoine, R., Broyer, M., Dugourd, P.: Specific photodissociation of peptides with multi-stage mass spectrometry. Rapid Comm. Mass Spectrom. 19, 2883–2892 (2005)
Girod, M., Zeljka, S., Marin, V., Rodolphe, A., Luke, M., Lemoine, J., Bonacic-Koutecky, V., Dugourd, P.: UV photodissociation of proline-containing peptide ions: insights from molecular dynamics. J. Am. Soc. Mass Spectrom. 26, 432–443 (2014)
Shaw, J.B., Li, W.Z., Holden, D.D., Zhang, Y., Griep-Raming, J., Fellers, R.T., Early, B.P., Thomas, P.M., Kelleher, N.L., Brodbelt, J.S.: Complete protein characterization using top-down mass spectrometry and ultraviolet photodissociation. J. Am. Chem. Soc. 135, 12646–12651 (2013)
Cannon, J.R., Carnmarata, M.B., Robotham, S.A., Cotham, V.C., Shaw, J.B., Fellers, R.T., Early, B.P., Thomas, P.M., Kelleher, N.L., Brodbelt, J.S.: Ultraviolet photodissociation for characterization of whole proteins on a chromatographic time scale. Anal. Chem. 86, 2185–2192 (2014)
Madsen, J., Cheng, R.R., Kaoud, T.S., Dalby, K.N., Makarov, D.E., Brodbelt, J.S.: Charge-site-dependent dissociation of hydrogen-rich radical peptide cations upon vacuum UV photoexcitation. Chem. Eur. J. 18, 5374–5383 (2012)
Thompson, M.S., Weidong, C.P., Reilly, J.P.: Fragmentation of singly charged peptide ions by photodissociation at lamda = 157 nm. Angew. Chem. Int. Ed. 43, 4791–4794 (2004)
Cui, W., Thompson, M.S., Reilly, J.P.: Pathways of peptide ion fragmentation induced by vacuum ultraviolet light. J. Am. Soc. Mass Spectrom. 16, 1384–1398 (2005)
Zhang, L., Cui, W., Thompson, M.S., Reilly, J.P.: Structures of α-type ions formed in the 157 nm photodissociation of singly-charged peptide ions. J. Am. Soc. Mass Spectrom. 17, 1315–1321 (2006)
Kim, T.-Y., Reilly, J.P.: Time-resolved observation of product ions generated by 157 nm photodissociation of singly protonated phosphopeptides. J. Am. Soc. Mass Spectrom. 20, 2334–2341 (2009)
Zubarev, R.A., Horn, D.M., Fridriksson, E.K., Kelleher, N.L., Kruger, N.A., Lewis, M.A., Carpenter, B.K., McLafferty, F.W.: Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 72, 563–573 (2000)
O’Connor, P.B., Lin, C., Cournoyer, J.J., Pittman, J.L., Belyayev, M., Budnik, B.A.: Long-lived electron capture dissociation product ions experience radical migration via hydrogen abstraction. J. Am. Soc. Mass Spectrom. 17, 576–585 (2006)
Kjeldsen, F., Silivra, O.A., Ivonin, I.A., Haselmann, K.F., Gorshkov, M., Zubarev, R.A.: Cα-C backbone fragmentation dominates in electron detachment dissociation of gas‐phase polypeptide polyanions. Chem. Eur. J. 11, 1803–1812 (2005)
Shaw, J.B., Madsen, J., Xu, H., Brodbelt, J.S.: Systematic comparison of ultraviolet photodissociation and electron transfer dissociation for peptide anion characterization. J. Am. Soc. Mass Spectrom. 23, 1707–1715 (2012)
Rumachik, N.G., McAlister, G.C., Russell, J.D., Bailey, D.J., Wenger, C.D., Coon, J.J.: Characterizing peptide neutral losses induced by negative electron-transfer dissociation (NETD). J. Am. Soc. Mass Spectrom. 23, 718–727 (2012)
Shaw, J.B., Kaplan, D.A., Brodbelt, J.S.: Activated ion negative electron transfer dissociation of multiply charged peptide anions. Anal. Chem. 85, 4721–4728 (2013)
Larraillet, V., Vorobyev, A., Brunet, C., Lemoine, J., Tsybin, Y.O., Antoine, R., Dugourd, P.: Comparative dissociation of peptide polyanions by electron impact and photo-induced electron detachment. J. Am. Soc. Mass Spectrom. 21, 670–680 (2010)
Larraillet, V., Antoine, R., Dugourd, P., Lemoine, J.: Activated-electron photodetachment dissociation for the structural characterization of protein polyanions. Anal. Chem. 81, 8410–8416 (2009)
Antoine, R., Joly, L., Tabarin, T., Broyer, M., Dugourd, P., Lemoine, J.: Photo-induced formation of radical anion peptides. Electron photodetachment dissociation experiments. Rapid Commun. Mass Spectrom. 21, 265–268 (2007)
Ganisl, B., Valovka, T., Hartl, M., Taucher, M., Bister, K., Breuker, K.: Electron detachment dissociation for top-down mass spectrometry of acidic proteins. Chem. Eur. J. 17, 4460–4469 (2011)
Turecek, F., Julian, R.R.: Peptide radicals and cation radicals in the gas phase. Chem. Rev. 113, 6691–6733 (2013)
Moore, B.N., Ly, T., Julian, R.R.: Radical conversion and migration in electron capture dissociation. J. Am. Chem. Soc. 133, 6997–7006 (2011)
Zubarev, R.: Peptide radical cations: gender determines dissociation chemistry. Mass Spectrom. (Tokyo, Japan) 2, S0004 (2013)
Coon, J.J., Shabanowitz, J., Hunt, D.F., Syka, J.E.P.: Electron transfer dissociation of peptide anions. J. Am. Soc. Mass Spectrom. 16, 880–882 (2005)
Oh, H.B., Moon, B.: Radical-driven peptide backbone dissociation tandem mass spectrometry. Mass Spectrom. Rev. 34, 116–132 (2015)
Sohn, C.H., Chung, C.K., Yin, S., Ramachandran, P., Loo, J.A., Beauchamp, J.L.: Probing the mechanism of electron capture and electron transfer dissociation using tags with variable electron affinity. J. Am. Chem. Soc. 131, 5444–5459 (2009)
Kalli, A., Hess, S.: Electron capture dissociation of hydrogen-deficient peptide radical cations. J. Am. Soc. Mass Spectrom. 23, 1729–1740 (2012)
Sun, Q., Nelson, H., Ly, T., Stoltz, B.M., Julian, R.R.: Side chain chemistry mediates backbone fragmentation in hydrogen deficient peptide radicals. J. Proteome Res. 8, 958–966 (2009)
Patiny, L., Borel, A.: ChemCalc: a building block for tomorrow’s chemical infrastructure. J. Chem. Inf. Model. 53, 1223–1228 (2013)
Frisch, M., Trucks, G., Schlegel, H.B., Scuseria, G., Robb, M., Cheeseman, J., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A. 02. Gaussian. Inc, Wallingford (2009)
Becke, A.D.: Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988)
Reed, A.E., Weinstock, R.B., Weinhold, F.: Natural population analysis. J. Chem. Phys. 83, 735–746 (1985)
Reed, A.E., Curtiss, L.A., Weinhold, F.: Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88, 899–926 (1988)
Runge, E., Gross, E.K.: Density-functional theory for time-dependent systems. Phys. Rev. Lett. 52, 997 (1984)
Han, X., Jin, M., Breuker, K., McLafferty, F.W.: Extending top-down mass spectrometry to proteins with masses greater than 200 kilodaltons. Science 314, 109–112 (2006)
Yoo, H.J., Ning, W., Shuyi, Z., Hangtian, S., Kristina, H.: Negative-ion electron capture dissociation: radical-driven fragmentation of charge-increased gaseous peptide anions. J. Am. Chem. Soc. 133, 16790–16793 (2011)
Madsen, J., Kaoud, T.S., Dalby, K.N., Brodbelt, J.S.: 193-nm Photodissociation of singly and multiply charged peptide anions for acidic proteome characterization. J. Proteom. 11, 1329–1334 (2011)
Bowie, J.H., Brinkworth, C.S., Dua, S.: Collision-induced fragmentations of the (M–H)− parent anions of underivatized peptides: an aid to structure determination and some unusual negative ion cleavages. Mass Spectrom. Rev. 21, 87–107 (2002)
Han, H., Xia, Y., McLuckey, S.A.: Ion trap collisional activation of c and z• ions formed via gas-phase ion/ion electron-transfer dissociation. J. Proteom. Res. 6, 3062–3069 (2007)
Zhang, L., Reilly, J.P.: Radical-driven dissociation of odd-electron peptide radical ions produced in 157 nm photodissociation. J. Am. Soc. Mass Spectrom. 20, 1378–1390 (2009)
Papayannopoulos, I.A.: The interpretation of collision-induced dissociation tandem mass spectra of peptides. Mass Spectrom. Rev. 14, 49–73 (1995)
Chu, I.K., Siu, C.K., Lau, J.K.C., Tang, W.K., Mu, X., Lai, C.K., Guo, X., Wang, X., Li, N., Xia, Y., Kong, X., Oh, H.B., Ryzhov, V., Tureček, F., Hopkinson, A.C., Siu, K.W.M.: Proposed nomenclature for peptide ion fragmentation. Int. J. Mass Spectrom. (2015). doi:10.1016/j.ijms.2015.07.021
Madsen, J.A., Xu, H., Robinson, M.R., Horton, A.P., Shaw, J.B., Giles, D.K., Kaoud, T.S., Dalby, K.N., Trent, M.S., Brodbelt, J.S.: High-throughput database search and large-scale negative polarity liquid chromatography–tandem mass spectrometry with ultraviolet photodissociation for complex proteomic samples. Mol. Cell. Proteom. 12, 2604–2614 (2013)
Fung, Y.M.E., Dominic, C.T.W.: Experimental and theoretical investigations of the loss of amino acid side chains in electron capture dissociation of model peptides. J. Am. Soc. Mass Spectrom. 16, 1523–1535 (2005)
Tureček, F., Syrstad, E.A.: Mechanism and energetics of intramolecular hydrogen transfer in amide and peptide radicals and cation-radicals. J. Am. Chem. Soc. 125, 3353–3369 (2003)
Cooper, H.J., Hudgins, R.R., Håkansson, K., Marshall, A.G.: Characterization of amino acid side chain losses in electron capture dissociation. J. Am. Soc. Mass Spectrom. 13, 241–249 (2002)
Harrison, A.G., Tu, Y.P.: Ion chemistry of protonated aspartic acid derivatives. J. Mass Spectrom. 33, 532–542 (1998)
Kim, T.-Y., Valentine, S.J., Clemmer, D.E., Reilly, J.P.: Gas-phase conformation-specific photofragmentation of proline-containing peptide ions. J. Am. Soc. Mass Spectrom. 21, 1455–1465 (2010)
Summerfield, S.G., Whiting, A., Gaskell, S.J.: Intra-ionic interactions in electrosprayed peptide ions. Int. J. Mass. Spectrom. Ion Process. 162, 149–161 (1997)
Sobolewski, A.L., Domcke, W., Dedonder-Lardeux, C., Jouvet, C.: Excited-state hydrogen detachment and hydrogen transfer driven by repulsive (1)pi sigma* states: a new paradigm for nonradiative decay in aromatic biomolecules. Phys. Chem. Chem. Phys. 4, 1093–1100 (2002)
Ashfold, M.N.R., King, G.A., Murdock, D., Nix, M.G.D., Oliver, T.A.A., Sage, A.G.: pi sigma* excited states in molecular photochemistry. Phys. Chem. Chem. Phys. 12, 1218–1238 (2010)
Ashfold, M.N.R., Cronin, B., Devine, A.L., Dixon, R.N., Nix, M.G.D.: The role of pi sigma* excited states in the photodissociation of heteroaromatic molecules. Science 312, 1637–1640 (2006)
Sage, A.G., Nix, M.G.D., Ashfold, M.N.R.: UV photodissociation of N-methylpyrrole: the role of pi sigma* states in non-hydride heteroaromatic systems. Chem. Phys. 347, 300–308 (2008)
Acknowledgments
The research leading to these results has received funding from the European Research Council under the European Union’s 7th Framework Program (FP7/2007-2013 grant agreement N°320659).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2577 kb)
Rights and permissions
About this article
Cite this article
Halim, M.A., Girod, M., MacAleese, L. et al. 213 nm Ultraviolet Photodissociation on Peptide Anions: Radical-Directed Fragmentation Patterns. J. Am. Soc. Mass Spectrom. 27, 474–486 (2016). https://doi.org/10.1007/s13361-015-1297-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1297-5