Abstract
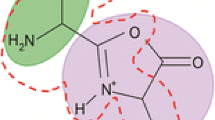
Infrared multiple-photon dissociation (IRMPD) spectroscopy and DFT calculations have been used to probe the most stable structures of a 3 * and a 4 * ions derived from both protonated pentaglycine (denoted G5) and pentaalanine (A5). The a 3 * and a 4 * ions derived from protonated A5 feature a CHR=N-CHR’- group at the N-terminus and an oxazolone ring at the C-terminus, as proposed previously [J. Am. Soc. Mass Spectrom. 19, 1788–1798 (2008)]. The isomeric a 4 * ion derived from A5 with a 3,5-dihydro-4H-imidazol-4-one ring structure was calculated to have a slightly better energy than the oxazolone, but the barrier to its formation is higher and there was no evidence of this ion in the IRMPD spectrum. By contrast, the a 4 * and [a 4 – H2O]+ (denoted a 4 0) ions from G5 gave strikingly similar IRMPD spectra and both have the 3,5-dihydro-4H-imidazol-4-one ring structure similar to that recently reported for the [GGGG + H – H2O]+ ion [Int. J. Mass Spectrom. 316–318, 268–272 (2012)]. In the absence of a solvent molecule, the pathway to the oxazolone is calculated to be lower than those to thermodynamically more stable products, the a 4 0 and the a 4 * with the 3,5-dihydro-4H-imidazol-4-one ring structure. Incorporation of one water molecule is sufficient to reduce the barrier to formation of the a 4 0 of G5 to below that for formation of the oxazolone. On the equivalent potential energy surface for protonated A5 the barrier to formation of the a 4 0 ion is 12.3 kcal mol–1 higher than that for oxazolone formation and the a 4 0 ion is not observed experimentally.

ᅟ









Similar content being viewed by others
References
Mann, M., Hendrickson, R.C., Pandey, A.: Analysis of proteins and proteomes by mass spectrometry. Annu. Rev. Biochem. 70, 437–473 (2001)
Aebersold, R., Goodlett, D.R.: Mass spectrometry in proteomics. Chem. Rev. 101, 269–295 (2001)
Savitski, M.M., Kjeldsen, F., Nielsen, M.L., Garbuzynskiy, S.O., Galzitskaya, O.V., Surin, A.K., Zubarev, R.A.: Backbone carbonyl group basicities are related to gas-phase fragmentation of peptides and protein folding. Angew. Chem., Int. Ed. 46, 1481–1484 (2007)
Yalcin, T., Khouw, K., Csizmadia, I.G., Peterson, M.R., Harrison, A.G.: Why are b ions stable species in peptide spectra? J. Am. Soc. Mass Spectrom. 6, 1165–1174 (1995)
Paizs, B., Suhai, S.: Fragmentation pathways of protonated peptides. Mass Spectrom. Rev. 24, 508–548 (2005)
Harrison, A.G.: To b or not to b: the ongoing saga of peptide b ions. Mass Spectrom. Rev. 28, 640–654 (2009)
Polfer, N.C., Oomens, J., Suhai, S., Paizs, B.: Spectroscopic and theoretical evidence for oxazolone ring formation in collison-induced dissociation of peptides. J. Am. Chem. Soc. 127, 17154–17155 (2005)
Chen, X.A., Steill, J.D., Oomens, J., Polfer, N.C.: Oxazolone versus macrocycle structures for leu-enkephalin b2–b4: insights from infrared multiple-photon dissociation spectroscopy and gas-phase hydrogen/deuterium exchange. J. Am. Soc. Mass Spectrom. 21, 1313–1321 (2010)
Polfer, N.C., Oomens, J., Suhai, S., Paizs, B.: Infrared spectroscopy and theoretical studies on gas-phase protonated leu-enkephalin and its fragments: direct experimental evidence for the mobile proton. J. Am. Chem. Soc. 129, 5887–5897 (2007)
Chen, X., Yu, L., Steill, J.D., Oomens, J., Polfer, N.C.: Effect of peptide fragment size on the propensity of cyclization in collision-induced dissociation: oligoglycine b2–b8. J. Am. Chem. Soc. 131, 18272–18282 (2009)
Erlekam, U., Bythell, B.J., Scuderi, D., Van Stipdonk, M., Paizs, B., Maitre, P.: Infrared spectroscopy of fragments of protonated peptides: direct evidence for macrocyclic structures of b5 ions. J. Am. Chem. Soc. 131, 11503–11508 (2009)
Saminathan, I.S., Wang, X.S., Guo, Y.Z., Krakovska, O., Voisin, S., Hopkinson, A.C., Siu, K.W.M.: The extent and effects of peptide sequence scrambling via formation of macrocyclic b ions in model proteins. J. Am. Soc. Mass Spectrom. 21, 2085–2094 (2010)
Golobrodko, A., Gorshko, M., Good, D., Zubarev, R.: Sequence scrambling in shotgun proteomics is negligible. J. Am. Soc. Mass Spectrom. 22, 1121–1124 (2011)
Yu, L., Tan, Y.L., Tsai, Y., Goodlett, D.R., Polfer, N.C.: On the relevance of peptide sequence permutations in shotgun proteomics studies. J. Proteome Res. 10, 2409–2416 (2011)
Yoon, S.H., Chamot-Rooke, J., Perkins, B.R., Hilderbrand, A.E., Poutsma, J.C., Wysocki, V.H.: IRMPD spectroscopy shows that AGG forms an oxazolone b 2 + ion. J. Am. Chem. Soc. 130, 17644–17645 (2008)
Oomens, J., Young, S., Molesworth, S., Van Stipdonk, M.: Spectroscopic evidence for an oxazolone structure of the b 2 fragment ion from protonated trialanine. J. Am. Soc. Mass Spectrom. 20, 334–339 (2009)
Yalcin, T., Harrison, A.G.: Ion chemistry of protonated lysine derivatives. J. Mass Spectrom. 31, 1237–1243 (1996)
Farrugia, J.M., Taverner, T., O’Hair, R.A.J.: Side-chain involvement in the fragmentation reactions of protonated methyl esters of histidine and its peptides. Int. J. Mass Spectrom. 209, 99–112 (2001)
Tsaprailis, G., Nair, H., Zhong, W., Kuppannan, K., Futrell, J.H., Wysocki, V.H.: A mechanistic investigation of the enhanced cleavage at histidine in the gas-phase dissociation of protonated peptides. Anal. Chem. 76, 2083–2094 (2004)
Bythell, B.J., Csonka, I.P., Suhai, S., Barofsky, D.F., Paizs, B.: Gas-phase structure and fragmentation pathways of singly protonated peptides with N-terminal arginine. J. Phys. Chem. B 114, 15092–15105 (2010)
Paizs, B., Szlávik, Z., Lendvay, G., Vékey, K., Suhai, S.: Formation of a 2 + ions of protonated peptides. An ab initio study. Rapid Commun. Mass Spectrom. 14, 746–755 (2000)
Vachet, R.W., Ray, K.L., Glish, G.L.: Origin of product ions in the MS/MS spectra of peptides in a quadrupole ion trap. J. Am. Soc. Mass Spectrom. 9, 341–344 (1998)
Ambihapathy, K., Yalcin, T., Leung, H.-W., Harrison, A.G.: Pathways to immonium ions in the fragmentation of protonated peptides. J. Mass Spectrom. 32, 209–215 (1997)
El Aribi, H., Rodriquez, C.F., Almeida, D.R.P., Ling, Y., Mak, W.W.-N., Hopkinson, A.C., Siu, K.W.M.: Elucidation of fragmentation mechanisms of protonated peptide ions and their products: a case study on glycylglycylglycine using density functional theory and threshold collision-induced dissociation. J. Am. Chem. Soc. 125, 9229–9236 (2003)
Verkerk, U.H., Siu, C.-K., Steill, J.D., Aribi, H.E., Zhao, J., Rodriquez, C.F., Oomens, J., Hopkinson, A.C., Siu, K.W.M.: a2 ion derived from triglycine: an N1-protonated 4-imidazolidinone. J. Phys. Chem. Lett. 1, 868–872 (2010)
Bythell, B.J., Maitre, P., Paizs, B.: Cyclization and rearrangement reactions of a n fragment ions of protonated peptides. J. Am. Chem. Soc. 132, 14766–14779 (2010)
Verkerk, U.H., Zhao, J., Lau, K.-C., Lam, T.-W., Hao, Q., Steill, J.D., Siu, C.-K., Oomens, J., Hopkinson, A.C., Siu, K.W.M.: Structures of the a 2 ions of Ala-Ala-Ala and Phe-Phe-Phe. Int. J. Mass Spectrom. 330/332, 254–261 (2012)
Bythell, B.J., Hernandez, O., Steinmetz, V., Paizs, B., Maitre, P.: Tyrosine side-chain catalyzed proton transfer in the YG a 2 ion revealed by theory and IR spectroscopy in the ‘fingerprint’ and X-H (X = C, N, O) stretching regions. Int. J. Mass Spectrom. 316/318, 227–234 (2012)
Yalcin, T., Csizmadia, I.G., Peterson, M.R., Harrison, A.G.: The structure and fragmentation of b n (n > 3) ions in peptide spectra. J. Am. Soc. Mass Spectrom. 7, 233–242 (1996)
Harrison, A.G., Young, A.B.: Fragmentation of protonated oligoalanines: amide bond cleavage and beyond. J. Am. Soc. Mass Spectrom. 15, 1810–1819 (2004)
Allen, J.M., Racine, A.H., Berman, A.M., Johnson, J.S., Bythell, B.J., Paizs, B., Glish, G.L.: Why are a 3 ions rarely observed? J. Am. Soc. Mass Spectrom. 19, 1764–1770 (2008)
Vachet, R.W., Bishop, B.M., Erickson, B.W., Glish, G.L.: Novel peptide dissociation: gas-phase intramolecular rearrangement of internal amino acid residues. J. Am. Chem. Soc. 119, 5481–5488 (1997)
Cooper, T., Talaty, E., Grove, J., Stipdonk, M., Suhai, S., Paizs, B.: Isotope labeling and theoretical study of the formation of a 3 * ions from protonated tetraglycine. J. Am. Soc. Mass Spectrom. 17, 1654–1664 (2006)
Bythell, B.J., Molesworth, S., Osburn, S., Cooper, T., Paizs, B., Van Stipdonk, M.: Structure and reactivity of a n and a n * peptide fragments investigated using isotope labeling, tandem mass spectrometry, and density functional theory calculations. J. Am. Soc. Mass Spectrom. 19, 1788–1798 (2008)
Polfer, N.C., Oomens, J.: Reaction products in mass spectrometry elucidated with infrared spectroscopy. Phys. Chem. Chem. Phys. 9, 3804–3817 (2007)
Marshall, A.G., Wang, T.C.L., Ricca, T.L.: Tailored excitation for Fourier-transform ion-cyclotron resonance mass spectrometry. J. Am. Chem. Soc. 107, 7893–7897 (1985)
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R., Montgomery, Jr., J. A., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H.; Hada, M., Ehara, M.; Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A.; Piskorz, P.; Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., and Pople, J. A., Gaussian, Inc., Wallingford CT.: Gaussian 03, revision D.01. Gaussian Inc., Wallingford CT (2004)
Becke, A.D.: Density functional theory thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Becke, A.D.: A new mixing of Hartree-Fock and local density functional theories. J. Chem. Phys. 98, 1372–1377 (1993)
Lee, C.T., Yang, W.T., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter 37, 785–789 (1998)
Hehre, W.J., Ditchfield, R., Pople, J.A.: Self-consistent molecular orbital methods: XII. Further extension of Gaussian basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972)
Hariharan, P.C., Pople, J.A.: The effect of d-functions on molecular orbital energies for hydrocarbons. Chem. Phys. Lett. 16, 217–219 (1972)
Chandrasekhar, J., Andrade, J.G., Schleyer, P.R.: Efficient and accurate calculation of anion proton affinities. J. Am. Chem. Soc. 103, 5609–5612 (1981)
Gonzalez, C., Schlegel, H.B.: An improved algorithm for reaction path following. J. Chem. Phys. 90, 2154–2161 (1989)
Steill, J., Zhao, J., Siu, C.-K., Ke, Y., Verkerk, U.H., Oomens, J., Dunbar, R.C., Hopkinson, A.C., Siu, K.W.M.: Structure of the observable histidine radical cation in the gas phase: a captodative α-radical ion. Angew. Chem. 47, 9666–9668 (2008)
Prell, J.S., Chang, T.M., Biles, J.A., Berden, G., Oomens, J., Williams, E.R.: Isomer population analysis of gaseous ions from infrared multiple photon dissociation kinetics. J. Phys. Chem. A 115, 2745–2751 (2011)
Verkerk, U.H., Zhao, J., Van Stipdonk, M.J., Bythell, B.J., Oomens, J., Hopkinson, A.C., Siu, K.W.M.: Structure of the [M + H – H2O]+ ion from tetraglycine: a revisit by means of density functional theory and isotope labeling. J. Phys. Chem. A 115, 6683–6687 (2011)
Lau, J.K.-C., Zhao, J., Siu, K.W.M., Hopkinson, A.C.: Elimination of water from the backbone of protonated tetraglycine. Inter. J. Mass Spectrom 316-318, 268–272 (2012)
Prell, J.S., O’Brien, J.T., Steill, J.D., Oomens, J., Williams, E.R.: Structures of protonated dipeptides: the role of arginine in stabilizing salt bridges. J. Am. Chem. Soc. 131, 11442–11449 (2009)
Forbes, M.W., Bush, M.F., Polfer, N.C., Oomens, J., Dunbar, R.C., Williams, E.R., Jockusch, R.A.: Infrared spectroscopy of arginine cation complexes: direct observation of gas-phase zwitterions. J. Phys. Chem. A 111, 11759–11770 (2007)
O’Brien, J.T., Prell, J.S., Steill, J.D., Oomens, J., Williams, E.R.: Interactions of mono- and divalent metal ions with aspartic and glutamic acid investigated with IR photodissociation spectroscopy and theory. J. Phys. Chem. A 112, 10823–10830 (2008)
Prell, J.S., Demireva, M., Williams, E.R.: Role of sequence in salt-bridge formation for alkali metal cationized GlyArg and ArgGly investigated with IRMPD spectroscopy and theory. J. Am. Chem. Soc. 131, 1232–1242 (2009)
Polfer, N.C., Oomens, J., Dunbar, R.C.: Alkali metal complexes of the dipeptides PheAla and AlaPhe: IRMPD Spectroscopy. Chem. Phys. Chem. 9, 579–589 (2008)
Paizs, B., Bythell, B.J., Maitre, P.: Rearrangement pathways of the a 4 ion of protonated YGGFL characterized by ir spectroscopy and modeling. J. Am. Soc. Mass Spectrom. 23, 664–675 (2012)
Bender, M.L.: Oxygen exchange as evidence for the existence of an intermediate in ester hydrolysis. J. Am. Chem. Soc. 73, 1626–1629 (1951)
Bender, M.L.: Intermediates in the reactions of carboxylic acid derivatives. II. Infrared absorption spectra as evidence for the formation of addition compounds of carboxylic acid derivatives. J. Am. Chem. Soc. 75, 5986–5990 (1953)
Dsouza, V.T., Bender, M.L.: Miniature organic models of enzymes. Acc. Chem. Res. 20, 146–152 (1987)
Acknowledgments
The authors acknowledge support for this study by the Natural Sciences and Engineering Research Council (NSERC) of Canada and made possible by the facilities of the Shared Hierarchical Academic Research Computing Network (http://www.sharcnet.ca) and the High Performance Computing Virtual Laboratory (http://www.hpcvl.org). Skillful assistance of the FELIX staff is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Schematic diagrams showing the formation of VII from XVII, the displacement of NH3 from the C-terminal protonated imine-amide by water creating the COOH group prior to formation of the five-membered ring, , the fragmentation of structures VII and VIII, the % parent ion depletion for Figures 1, 3, 4, 5 and 6, the CID spectrum of a 4 ion derived from G 5 , structure V diastereomer formation, the full reference for Gaussian 03, Revision D, and total energies and Cartesian coordinates of the optimized structures at the B3LYP/6-311++G(d,p) level of theory. (PDF 554 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Lau, J.KC., Grzetic, J. et al. Structures of a n * Ions Derived from Protonated Pentaglycine and Pentaalanine: Results from IRMPD Spectroscopy and DFT Calculations. J. Am. Soc. Mass Spectrom. 24, 1957–1968 (2013). https://doi.org/10.1007/s13361-013-0728-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0728-4