Abstract
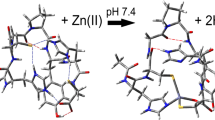
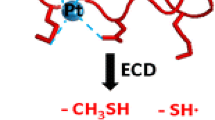
The binding preferences of Pb2+and Zn2+ in doubly charged complexes with zinc finger-like 12-residue peptides (Pep), [Mn(Pep-2(n-1)H)]2+ have been explored using tandem mass spectrometry. The peptides were synthesized strategically by blocking the N-terminus with an acetyl group and with four cysteine and/or histidine residues in positions 2, 5, 8, and 11, arranged in different motifs: CCHH, CHCH, and CCCC. The MS2 spectra of the Pb2+ and Zn2+ complexes show multiple losses of water and a single methane loss and these provide a sensitive method for locating the metal dication and so elucidating its coordination. The elimination of a methane molecule indicated the position of the metal at the Cys2 residue. Whereas lead was observed to preferentially bind to cysteine residues, zinc was found to primarily bind to histidine residues and secondarily to cysteine residues. Preferential binding of lead to cysteine is preserved in the complexes with more than one Pb2+. Key to the mechanism of the loss of water and methane is the metal dication withdrawing electrons from the proximal amidic nitrogen. This acidic nitrogen loses its hydrogen to an amidic oxygen situated four atoms away leading to formation of a five-member ring and the elimination of water.








Similar content being viewed by others
References
Haraguchi, H.: Metallomics as integrated biometal science. J. Anal. At. Spectrom. 19, 5–14 (2004)
Seregin, I.V., Ivanov, V.B.: Physiological aspects of cadmium and lead toxic effects on higher plants. Russ. J. Plant Physiol. 48, 523–544 (2001)
Cerklewski, F.L., Forbes, R.M.: Influence of dietary zinc on lead toxicity in the rat. J. Nutr. 106(5), 689–696 (1976)
Vallee, B.L., Ulmer, D.D.: Biochemical effects of mercury, cadmium, and lead. Annu Rev. Biochem. 41, 91–128 (1972)
Payne, C.J., Horst, A.M., Godwin, A.H.: Lead fingers: Pb2+ binding to structural zinc-binding domains determined directly by monitoring lead − thiolate charge-transfer bands. J. Am. Chem. Soc. 121, 6850–6855 (1999)
International Human Genome Sequencing Consortium: Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001)
Wolfe, S.A., Necludova, L., Pabo, C.A.: DNA recognition by Cys 2 His 2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 29, 181–212 (2000)
Schwabe, W.R.J., Klug, A.: Zinc mining for protein domains. Nat. Struct. Biol. 1, 345–349 (1994)
Pavletich, N.P.: Pabo, C.O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA Complex at 2.1 A. Science 252, 809–817 (1991)
Blake, P.R., Summers, M.F.: The amino-terminal residues X(i − 1)-C(i)-X(i + 1)-X(i + 2)-C(i + 3)-G(i + 4)-X(i + 5) form a metal-coordinating reverse turn. Adv. Biophys. Chem. 4, 1–30 (1994)
Simonson, T., Calimet, N.: CysxHisy–Zn2+ interactions: Possibilities and limitations of a simple pairwise force field. J. Molec. Graph. Modeling 24, 404–411 (2006)
Murariu, M., Dragan, S.E., Drochioiu, G.: Synthetic cysteine-peptide and its relation with heavy and radioactive metals. Biopolymers 93(6), 497–508 (2010)
Godwin, H.A.: The biological chemistry of lead. Curr Opin. Chem. Biol. 5, 223–227 (2001)
Golden, M.L., Reibenspies, J.H., Darensbourg, M.Y.: Accommodation of the irregular coordination geometry of lead(II) by a square planar N2S2 ligand and its preference for zinc(II). Inorg. Chem. 43, 5798–5800 (2004)
Andersen, R.J.: diTargiani, R.C., Hancock, R.D., Stern, C.L., Goldberg, D.P., Godwin, H.A. Characterization of the first N2S(alkylthiolate)lead compound: a model for three-coordinate lead in biological systems. Inorg. Chem. 45, 6574–6576 (2006)
Magyar, J.S., Weng, T.-C., Stern, C.M., Dye, D.F., Rous, B.W., Pyne, J.C., Bridgewater, B.M., Mijovilovich, A., Parkin, G., Zaleski, J.M., Panner-Hahn, J.E., Godwin, H.A.: Re-examination of lead(II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning. J. Am. Chem. Soc. 127, 9495–9505 (2005)
Bridgewater, B.M., Parkin, G.: Lead poisoning and the inactivation of 5-aminolevulinate dehydratase as modeled by the Tris(2-mercapto-1-phenylimidazolyl)hydroborato lead complex, {[TmPh]Pb}[ClO4]. J. Am. Chem. Soc. 122, 7140–7144 (2000)
Pieper, S., Beck, S., Ahrends, R., Scheler, C., Linscheid, M.W.: Fragmentation behavior of metal-coded affinity tag (MeCAT)-labeled peptides. Rapid Commun. Mass Spectrom. 2009(23), 2045–2052 (2009)
Greogorius, B., Schaumloffel, D., Hildebrandt, A., Tholey, A.: Characterization of metal-labeled peptides by matrix-assisted laser desorption/ionization mass spectrometry and tandem mass spectrometry. Rapid. Commun. Mass Spectrom. 24, 3279–3289 (2010)
Chu, I.K., Guo, X., Lau, T.-C., Siu, M.K.W.: Sequencing of argentinated peptides by means of electrospray tandem mass spectrometry. Anal. Chem. 71, 2364–2372 (1999)
Thorne, C.G., Ballard, D.K., Gaskell J.S. Metastable decomposition of peptide [M + H]+ ions via rearrangement involving loss of the c-terminal amino-acid residue. J. Am. Soc. Mass Spectrom. 1, 249–257 (1990)
Teesch, M.L., Adams, J.: Fragmentations of gas-phase complexes between alkali metal ions and peptides: metal ion binding to carbonyl oxygens and other neutral functional groups. J. Am. Chem. Soc. 113, 812–820 (1991)
Teesch, M.L., Orlando, C.R., Adams, J.: Location of the alkali metal ion in gas-phase peptide complexes. J. Am. Chem. Soc. 113, 3668–3675 (1991)
Chu, K.I., Cox, D.M., Guo, X., Kireeva, I., Lau, T.C., McDermott, J.C., Siu, K.W.M.: Sequencing of argentinated peptides by means of matrix-assisted laser desorption/ionization tandem mass spectrometry. Anal. Chem. 74, 2072–2082 (2002)
Kim, B.-R., Kim, H.-T.: Gas-phase investigation of [(Cu2+, Ni2+ – Gly-Gly-His) – 3H+]–1 complex by electrospray ionization MS/MS and MS/MS/MS. Bull. Korean Chem. Soc. 28(5), 840–842 (2007)
Pu, D., Vincent, B.J., Cassady, J.C.: The effects of chromium(III) coordination on the dissociation of acidic peptides. J. Mass Spectrom. 43, 773–781 (2008)
Shi, T., Siu, K.W.M., Hopkinson, A.: Generation of [La(peptide)]3+ complexes in the gas phase: determination of the number of binding sites provided by dipeptide, tripeptide, and tetrapeptide ligands. J. Phys. Chem. A 111, 11562–11571 (2007)
Hu, P., Loo, A.J.: Gas-phase coordination properties of Zn2+, Cu2+, Ni2+, and Co2+ with histidine-containing peptides. J. Am. Chem. Soc. 117, 11314–11319 (1995)
Ngoka, L.C.M., Gross, M.L.: Location of alkali metal binding sites in endothelinA selective receptor antagonists, cyclo(D-Trp–D-Asp–Pro–D-Val–Leu) and cyclo(D-Trp–D-Asp–Pro–D-Ile–Leu), from multistep collisionally activated decompositions. J. Mass Spectrom. 35, 265–276 (2000)
Williams, S., Brodbelt, J.S.: MSn characterization of protonated cyclic peptides and metal complexes. J. Am. Soc. Mass Spectrom. 15, 1039–1054 (2004)
Lavanant, H., Hoppilliard, Y.: Fragmentation of arginine- and lysine-containing dipeptides cationized by Cu+ and Cu2+. Eur. Mass Spectrom. 5, 41–50 (1999)
Loo, A.J., Hu, P., Smith, R.: Interaction of angiotensin peptides and zinc metal ions probed by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 5, 959–965 (1994)
Grese, R.P., Cerny, L.R., Gross, M.L.: Metal ion–peptide interactions in the gas phase: a tandem mass spectrometry study of alkali metal cationized peptides. J. Am. Chem. Soc. 111, 2835–2842 (1989)
Tang, X.-J., Thibault, P., Boyd, R.K.: Fragmentation reactions of singly and doubly charged alkali metal ion–peptide complexes: a reaction specific to C-terminal arginine residues. Organic Mass Spectrom. 28, 1047–1052 (1993)
Udo, V., Zhao, J., Van Stipdonk, M., Bythell, B., Oomens, J., Hopkinson, C.A., Siu, K.W.M.: Structure of the [M + H – H2O]+ Ion from tetraglycine: a revisit by means of density functional theory and isotope labeling. J. Phys. Chem. 115, 6683–6687 (2011)
Hu, P.F., Gross, M.L.: Gas-phase interactions of transition-metal ions and di- and tripeptides: a comparison with alkaline-earth-metal-ion interactions. J. Am. Chem. Soc. 115, 8821–8828 (1993)
Hu, P.F., Gross, M.L.: Strong interactions of anionic peptides and alkaline earth metal ions: bis(peptide) complexes in the gas phase. J. Am. Chem. Soc. 114, 9161–9169 (1992)
Reid, G.E., Simpson, R.J., O’Hair, R.A.J.: A mass spectrometric and ab initio study of the pathways for dehydration of simple glycine and cysteine-containing peptide [M + H]+ ions. J. Am. Soc. Mass Spectrom. 9, 945–956 (1998)
Sabareesh, V., Balaram, P.: Tandem electrospray mass spectrometric studies of proton and sodium ion adducts of neutral peptides with modified N- and C-termini: synthetic model peptides and microheterogeneous peptaibol antibiotics. Rapid Commun. Mass Spectrom. 20, 618–628 (2006)
Acknowledgments
The authors greatly appreciate continued funding from the Natural Sciences and Engineering Research Council of Canada. As holder of a Canada Research Chair in Physical Chemistry, D.K.B. thanks the contributions of the Canada Research Chair Program to this research.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 200 kb)
Rights and permissions
About this article
Cite this article
Banu, L., Blagojevic, V. & Bohme, D.K. Locating Pb2+ and Zn2+ in Zinc Finger-Like Peptides Using Mass Spectrometry. J. Am. Soc. Mass Spectrom. 24, 1534–1542 (2013). https://doi.org/10.1007/s13361-013-0682-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0682-1