Abstract
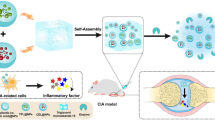
Tuberculosis (TB) is a potentially fatal contagious disease and is a second leading infectious cause of death in the world. Osteoarticular TB is treated using standard regimen of 1st and 2nd line anti-tubercular drugs (ATDs) for extensive period of 8–20 months. These drugs are commonly administered in high doses by oral route or by intravenous route, because of their compromised bioavailability. The common drawbacks associated with conventional therapy are poor patient compliance due to long treatment period, frequent and high dosing, and toxicity. This aspect marks for the need of formulations to eliminate these drawbacks. MTB is an intracellular pathogen of mononuclear phagocyte. This attribute makes nanotherapeutics an ideal approach for MTB treatment as macrophages capture nano forms. Polymeric nanoparticles are removed from the body by opsonization and phagocytosis, which forms an ideal strategy to target macrophage containing mycobacteria. To further improve targetability, the nanoparticles are conjugated with ligand, which serves as an easy substrate for the receptors present on the macrophage surface. The purpose of present work was to develop intra-articular injectable in situ gelling system containing polymeric nanoparticles, which would have promising advantages over conventional method of treatment. The rationale behind formulating nanoparticle incorporated in situ gel-based system was to ensure localization of the formulation in intra-articular cavity along with sustained release and conjugation of nanoparticles with mannose as ligand to improve uptake by macrophages. Rifampicin standard ATD was formulated into chitosan nanoparticles. Chitosan with 85% degree of deacetylation (DDA) and sodium tripolyphosphate (TPP) as the crosslinking agent was used for preparing nanoparticles. The percent entrapment was found to be about 71%. The prepared nanoparticles were conjugated with mannose. Conjugation of ligand was ascertained by performing Fourier transformed infrared spectroscopy. The particle size was found to be in the range of 130–140 nm and zeta potential of 38.5 mV. Additionally, we performed scanning electron microscopy to characterize the surface morphology of ligand-conjugated nanoparticles. The conjugated chitosan nanoparticles were incorporated into in situ gelling system comprising Poloxamer 407 and HPMC K4M. The gelling system was evaluated for viscosity, gelling characteristics, and syringeability. The drug release from conjugated nanoparticles incorporated in in situ gel was found to be about 70.3% at the end of 40 h in simulated synovial fluid following zero-order release kinetics. Based on the initial encouraging results obtained, the nanoparticles are being envisaged for ex vivo cellular uptake study using TB-infected macrophages
Graphical abstract







Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TB:
-
Tuberculosis
- BJTB:
-
Bone and joint tuberculosis
- MTB:
-
Mycobacterium
- ATDs:
-
Anti-tubercular drugs
- TPP:
-
Sodium tripolyphosphate
- STAB:
-
Sodium triacetoxy borohydride
- HPMC:
-
Hydroxy propyl methyl cellulose
- DDA:
-
Degree of deacetylation
- %EE:
-
Percent entrapment efficiency
- FTIR:
-
Fourier transformed infrared spectroscopy
- DSC:
-
Differential scanning calorimetry
- MCNPs:
-
Mannose-conjugated chitosan nanoparticles
- PDI:
-
Polydispersity index
References
Bhowmik CD, Chandira RM, Jayakar B, Kumar KPS. Recent trends of drug used treatment of tuberculosis. J Chem Pharm Res. 2009;1(1):113–33.
Davidson PT, Horowitz I. Skeletal tuberculosis. A review with patient presentations and discussion. Am J Med. 1970;48(1):77–84.
Sequeira W, Co H, Block J. Osteoarticular tuberculosis: current diagnosis and treatment - PubMed. Am J Ther. 2000;7(6):393–8.
Procopie I, Popescu EL, Huplea V, Pleșea RM, Ghelase ȘM, Stoica GA, et al. Osteoarticular tuberculosis-brief review of clinical morphological and therapeutic profiles. Curr Heal Sci J. 2017;43(3):171–90.
Tuli SM, Brighton CT, Morton HE, Clark LW. The experimental induction of localised skeletal tuberculous lesions and their accessibility to streptomycin. J Bone Jt Surg - Ser B. 1974;56(3):551–9.
Pandita A, Madhuripan N, Pandita S, Hurtado RM. Challenges and controversies in the treatment of spinal tuberculosis. J Clin Tuber Other Mycobact Dis. 2020;1(19):100151.
Diseases C. WHO Report 2003 Global Tuberculosis Control. 2003; Accessed 30 March 2021
Sawarkar SP. Targeting approaches for effective therapeutics of bone tuberculosis abstract symptoms of bone tuberculosis. J Pharm Microbiol. 2017;3(1):1–3.
Chan ED, Iseman MD. Clinical review Current medical treatment for tuberculosis. Br Med J. 2002;325(7375):1282–6.
Organization WH. Anti-tuberculosis drug resistance in the world Third Global Report The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance [Internet]. 1999 [cited 2021 Mar 30].
Sosnik A, Glisoni R, Moretton MA, Carcaboso ÁM, Glisoni RJ, Chiappetta DA. New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Adv Drug Deliv Rev. 2009;62:547–59.
Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009;50(1):1–11.
Ramón-García S, Mikut R, Ng C, Ruden S, Volkmer R, Reischl M, et al. Targeting Mycobacterium tuberculosis and other microbial pathogens using improved synthetic antibacterial peptides. Am Soc Microbiol. 2013;57(5):2295–303.
Stahl PD. The mannose receptor and other macrophage lectins. Curr Biol. 1992;2(4):183.
Rajaram MVS, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor γ linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185(2):929–42.
Rajaram MVS, Arnett E, Azad AK, Guirado E, Ni B, Gerberick AD, et al. M. tuberculosis-initiated human mannose receptor signaling regulates macrophage recognition and vesicle trafficking by FcRγ-chain, Grb2, and SHP-1. Cell Rep. 2017;21(1):126–40.
Pawde DM, Viswanadh MK, Mehata AK, Sonkar R, Narendra, Poddar S, et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm J. 2020;28(12):1616–25.
Chaubey P, Mishra B. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr Polym. 2014;101(1):1101–8.
Chaubey P, Patel RR, Mishra B. Development and optimization of curcumin-loaded mannosylated chitosan nanoparticles using response surface methodology in the treatment of visceral leishmaniasis. Expert Opin Drug Deliv. 2014;11(8):1163–81.
Abdelgawad AM, Hudson SM. Chitosan nanoparticles: polyphosphates cross-linking and protein delivery properties. Int J Biol Macromol. 2019;136:133–42.
Goñi MG, Tomadoni B, Roura SI, del Rosario Moreira M. Lactic acid as potential substitute of acetic acid for dissolution of chitosan: preharvest application to butterhead lettuce. J Food Sci Technol. 2017;54(3):620–6.
Vandervoort J, Ludwig A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur J Pharm Biopharm. 2004;57(2):251–61.
Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28.
Rabel M, Warncke P, Grüttner C, Bergemann C, Kurland HD, Müller R, et al. Simulation of the long-term fate of superparamagnetic iron oxide-based nanoparticles using simulated biological fluids. Nanomedicine. 2019;14(13):1681–706.
Thing M, Mertz N, Ågårdh L, Larsen SW, Østergaard JLC. Simulated synovial fluids for in vitro drug and prodrug release testing of depot injectables intended for joint injection. J Drug Deliv Sci Technol. 2019;49(1):169–76.
Talasaz AHH, Ghahremankhani AA, Moghadam SH, Malekshahi MR, Atyabi F, Dinarvand R. In situ gel forming systems of poloxamer 407 and hydroxypropyl cellulose or hydroxypropyl methyl cellulose mixtures for controlled delivery of vancomycin. J Appl Polym Sci. 2008;109(4):2369–74.
Levy G, Amherst NY. Kinetics of drug action: an overview. J Allergy Clin Immunol. 1985;78(4(2)):754–61.
Franzblau SG, Witzig RS, Mclaughlin JC, Torres P, Madico G, Hernandez A, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue Assay. J Clin Microbiol. 1998;36
Gail L. Woods, Barbara A. Brown-Elliott, Patricia S. Conville, Edward P. Desmond, Geraldine S. Hall, Grace Lin, et al. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic actinomycetes - PubMed. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute, editor. CLSI Standards: Guidelines for Health Care Excellence. 2011.
Kaur P, Ghosh A, Krishnamurthy RV, Bhattacharjee DG, Achar V, Datta S, et al. A High-Throughput Cidality Screen for Mycobacterium Tuberculosis. Manganelli R, editor. PLoS One. 2015;10(2):e0117577.
Drapeau CMJ, Grilli E, Petrosillo N. Rifampicin combined regimens for Gram-negative infections: data from the literature. Int J Antimicrob Agents. 2010;35(1):39–44.
Kumar PV, Asthana A, Dutta T, Jain NK. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006;14(8):546–56.
Patel BK, Parikh RH, Aboti PS. Development of oral sustained release Rifampicin loaded chitosan nanoparticles by design of experiment. J Drug Deliv. 2013;2013:1–10.
Lazaridou M, Christodoulou E, Nerantzaki M, Kostoglou M, Lambropoulou DA, Katsarou A, et al. Formulation and in-vitro characterization of chitosan-nanoparticles loaded with the iron chelator deferoxamine mesylate (DFO). Pharmaceutics. 2020;12(3).
Mayol L, Quaglia F, Borzacchiello A, Ambrosio L, Rotonda MIL. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: rheological, mucoadhesive and in vitro release properties. Eur J Pharm Biopharm. 2008;70(1):199–206.
Radivojša M, Grabnar I, Grabnar PA. Thermoreversible in situ gelling poloxamer-based systems with chitosan nanocomplexes for prolonged subcutaneous delivery of heparin: design and in vitro evaluation. Eur J Pharm Sci. 2013;50(1):93–101.
Acknowledgements
The authors wish to thank Sophisticated Analytical Instrument Facility, IIT-Bombay, Powai for their providing SEM analysis samples which are used in this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study and manuscript conception and design. Material preparation, data collection, and analysis and experiments were performed by Ms Pratiksha Prabhu and Ms. Trinette Fernandes. The research activity planning, execution, and supervision were carried out under the guidance of Dr Sujata Sawarkar and Dr Pramila Chaubey. Dr. Shridhar Dr Ramya VK and Dr. Parminder Kaur conducted the MIC studies of formulation against M. tb. All authors commented on previous versions of the manuscript, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prabhu, P., Fernandes, T., Chaubey, P. et al. Mannose-conjugated chitosan nanoparticles for delivery of Rifampicin to Osteoarticular tuberculosis. Drug Deliv. and Transl. Res. 11, 1509–1519 (2021). https://doi.org/10.1007/s13346-021-01003-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01003-7