Abstract
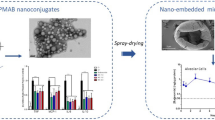
The co-existence with rhinitis limits the control of asthma. Compared with oral H1 receptor antagonists, intranasal corticosteroids have been demonstrated to provide greater relief of all symptoms of rhinitis and are recommended as first-line treatment for allergic rhinitis. Intrinsic limitations of nasal delivery, such as the presence of the protective mucous layer, the relentless mucociliary clearance, and the consequent reduced residence time of the formulation in the nasal cavity, limit budesonide efficacy to the treatment of local nasal symptoms. To overcome these limitations and to enable the treatment of asthma via nasal administration, we developed a budesonide-loaded lipid-core nanocapsule (BudNC) microagglomerate powder by spray-drying using a one-step innovative approach. BudNC was obtained, as a white powder, using L-leucine as adjuvant with 75 ± 6% yield. The powder showed a bimodal size distribution curve by laser diffraction with a principal peak just above 3 μm and a second one around 0.45 μm and a drug content determined by HPLC of 8.7 mg of budesonide per gram. In vivo after nasal administration, BudNC showed an improved efficacy in terms of reduction of immune cell influx; production of eotaxin-1, the main inflammatory chemokine; and arrest of airways remodeling when compared with a commercial budesonide product in both short- and long-term asthma models. In addition, data showed that the results in the long-term asthma model were more compelling than the results obtained in the short-term model.

Graphical abstract









Similar content being viewed by others
References
Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–54.
Fixman ED, Stewart A, Martin JG. Basic mechanisms of development of airway structural changes in asthma. Eur Respir J. 2007;29:379–89.
Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–97.
Mullol J, Valero A, Alobid I, Bartra J, Navarro AM, Chivato T, et al. Allergic rhinitis and its impact on asthma update (ARIA 2008), the perspective from Spain. J Investig Allergol Clin Immunol. 2008;18(5):327–34.
Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy. 2013;68:569–79.
Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317:1624–9.
Kumar A, Pandey AN, Jain SK. Nasal nanotechnology: revolution for efficient therapeutics delivery. Drug Deliv. 2014;23:671–83.
Pelaia G, Vatrella A, Busceti MT, Fabiano F, Terracciano R, Matera MG, et al. Molecular and cellular mechanisms underlying the therapeutic effects of budesonide in asthma. Pulm Pharmacol Ther. 2016;40:15–21.
Stanaland BE. Once-daily budesonide aqueous nasal spray for allergic rhinitis: a review. Clin Ther. 2004;26(4):473–92.
Tiozzo Fasiolo L, Manniello MD, Tratta E, Buttini F, Rossi A, Sonvico F, et al. Opportunity and challenges of nasal powders: drug formulation and delivery. Eur J Pharm Sci. 2018;113:2–17.
Sonvico F, Clementino A, Buttini F, Colombo G, Pescina S, Guterres SS, et al. Surface-modified nanocarriers for nose-to-brain delivery: from bioadhesion to targeting. Pharmaceutics. 2018;10. https://doi.org/10.3390/pharmaceutics10010034.
Fiel LA, Adorne MD, Guterres SS, Netz PA, Pohlmann AR. Variable temperature multiple light scattering analysis to determine the enthalpic term of a reversible agglomeration in submicrometric colloidal formulations: a quick quantitative comparison of the relative physical stability. Colloids Surf A Physicochem Eng Asp. 2013;431:93–104.
Jager E, Venturini CG, Poletto FS, Colome LM, Pohlmann JP, Bernardi A, et al. Sustained release from lipid-core nanocapsules by varying the core viscosity and the particle surface area. J Biomed Nanotechnol. 2009;5:130–40.
Venturini CG, Jager E, Oliveira CP, Bernardi A, Battastini AMO, Guterres SS, et al. Formulation of lipid core nanocapsules. Colloids Surf A Physicochem Eng Asp. 2011;375:200–8.
Guterres SS, Beck RCR, Pohlmann AR. Spray-drying technique to prepare innovative nanoparticulated formulations for drug administration: a brief overview. Braz J Phys. 2009;39:205–9.
Deb U, Lomash V, Raghuvanshi S, Pant SC, Vijayaraghavan R. Effects of 28 days silicon dioxide aerosol exposure on respiratory parameters, blood biochemical variables and lung histopathology in rats. Environ Toxicol Pharmacol. 2012;34(3):977–84.
Guterres SS, Pohlmann AR, Beck RCR, Dimer FA, Ortiz M. Processo one-pot de síntese de nanocápsulas poliméricas, suas formas secas, composições farmacêuticas de nanocápsulas poliméricas e seus usos. Patent application INPI BR 102013019136-1 A2; 2013.
Tewa-Tagne P, Briancon S, Fessi H. Preparation of redispersible dry nanocapsules by means of spray-drying: development and characterization. Eur J Pharm Sci. 2007;30:124–35.
El-Gendy N, Gorman EM, Munson EJ, Berkland C. Budesonide nanoparticle agglomerates as dry powder aerosols with rapid dissolution. J Pharm Sci. 2009;98:2731–46.
Coutinho DS, Anjos-Valotta EA, do Nascimento C, Pires ALA, Napimoga MH, Carvalho VF, et al. 15-Deoxy-Delta-12,14-Prostaglandin J2 Inhibits Lung Inflammation and Remodeling in Distinct Murine Models of Asthma. Front. Immunol. 2017;8:740.
Serra MF, Anjos-Valotta EA, Olsen PC, Couto GC, Jurgilas PB, Cotias AC, et al. Nebulized lidocaine prevents airway inflammation, peribronchial fibrosis, and mucus production in a murine model of asthma. Anesthesiology. 2012;117:580–91.
Subhashini CPS, Kumari S, Kumar JP, Chawla R, Dash D, Singh M, et al. Intranasal curcumin and its evaluation in murine model of asthma. Int Immunopharmacol. 2013;17(3):733–43.
Wu W, Chen H, Li YM, Wang SY, Diao X, Liu KG. Intranasal siRNA targeting c-kit reduces airway inflammation in experimental allergic asthma. Int J Clin Exp Pathol. 2014;7(9):5505–14.
Beck RCR, Ourique AF, Guterres SS, Pohlmann AR. Spray-dried polymeric nanoparticles for pharmaceutics: a review of patents. Recent Pat Drug Deliv Formul. 2012;6:195–208.
Jornada DS, Fiel LA, Bueno K, Gerent JF, Petzhold CL, Beck RCR, et al. Lipid-core nanocapsules: mechanism of self-assembly, control of size and loading capacity. Soft Matter. 2012;8:6646–55.
Najafabadi AR, Gilani K, Barghi M, Rafiee-Tehrani M. The effect of vehicle on physical properties and aerosolisation behaviour of disodium cromoglycate microparticles spray dried alone or with L-leucine. Int J Pharm. 2004;285:97–108.
Li FQ, Yan C, Bi J, Lv WL, Ji RR, Chen X, et al. A novel spray-dried nanoparticles-in-microparticles system for formulating scopolamine hydrobromide into orally disintegrating tablets. Int J Nanomedicine. 2011;6:897–904.
Vehring R, Foss WR, Lechuga-Ballesteros D. Particle formation in spray drying. J Aerosol Sci. 2007;38:728–46.
Carvalho TC, Peters JI, Williams RO 3rd. Influence of particle size on regional lung deposition-what evidence is there? Int J Pharm. 2011;406:1–10.
Sosnik A. das Neves J, Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog Polym Sci. 2014;39(12):2030–75.
Gueders, et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 2009;58:845–54.
De Sanctis GT, Merchant M, Beier DR, Dredge RD, Grobholz JK, Martin TR, et al. Quantitative locus analysis of airway hyperresponsiveness in A/J and C57BL/6J mice. Nat Genet. 1995;11:150–4.
Serra MF, Cotias AC, Pao CRR, Daleprane JB, Jurgilas PB, Couto GC, et al. Repeated allergen exposure in A/J mice causes steroid-insensitive asthma via a defect in glucocorticoid receptor bioavailability. J Immunol. 2018;201:851–60.
Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003;168:959–67.
Matucci A, Maggi E, Vultaggio A. Eosinophils, the IL-5/IL-5Ralpha axis, and the biologic effects of benralizumab in severe asthma. Respir Med. 2019;160:105819.
Foster PS, Ming Y, Matthei KI, Young IG, Temelkovski J, Kumar RK. Dissociation of inflammatory and epithelial responses in a murine model of chronic asthma. Lab Investig. 2000;80(5):655–62.
Dent G, Hadjicharalambous C, Yoshikawa T, Handy RL, Powell J, Anderson IK, et al. Contribution of eotaxin-1 to eosinophil chemotactic activity of moderate and severe asthmatic sputum. Am J Respir Crit Care Med. 2004;169(10):1110–7.
Nair P, O'Byrne PM. The interleukin-13 paradox in asthma: effective biology, ineffective biologicals. Eur Respir J. 2019;53(2):1802250.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98.
Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70(8):748–56.
Bai TR. Evidence for airway remodeling in chronic asthma. Curr Opin Allergy Clin Immunol. 2010;10:82–6.
Holgate ST, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet. 2006;368:780–93.
Walsh ER, Stokes K, August A. The role of eosinophils in allergic airway inflammation. Discov Med. 2010;9:357–62.
Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710.
Chaves PS, Frank LA, Frank AG, Pohlmann AR, Guterres SS, Beck RC. Mucoadhesive properties of Eudragit®RS100, Eudragit®S100, and poly(ε-caprolactone) nanocapsules: influence of the vehicle and the mucosal surface. AAPS PharmSciTech. 2018;19(4):1637–46.
Funding
This study is part of the National Institute of Science and Technology in Pharmaceutical Nanotechnology: a transdisciplinary approach (INCT-NANOFARMA), which is supported by São Paulo Research Foundation (FAPESP, Brazil) Grant no.2014/50928-2, and by the Brazilian National Council for Scientific and Technological Development (CNPq, Brazil) Grant no. 465687/2014-8. The project is also supported by the Research Support Foundation of the State of Rio Grande do Sul (PRONEX FAPERGS/CNPq 12/2014 #16/2551-0000467-6) and Research Support Foundation of the State of Rio de Janeiro (Grant no. E-26/010.000983/2019)(NanoHEALTH). Professors Guterres, S.S., Pohlmann AR, and Martins MA are recipient of Productivity Grants in CNPq.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Diego de Sa Coutinho, Bianca Torres Ciambarella, Everton Tenorio de Souza, Ana Paula Leite D’Almeida, Taís Lusa Durli, Patrícia Machado Rodrigues e Silva, Andressa Bernardi, Fabio Sonvico, and Marco Aurelio Martins declare that they have no conflict of interest. Manoel Ortiz, Adriana Raffin Pohlmann, and Silvia Stanisçuaski Guterres have the potential conflict of interest because of a patent application related to the topic of the study (INPI BR 102013019136-1 A2; 2013).
Ethics statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ortiz, M., de Sa Coutinho, D., Ciambarella, B.T. et al. Intranasal administration of budesonide-loaded nanocapsule microagglomerates as an innovative strategy for asthma treatment. Drug Deliv. and Transl. Res. 10, 1700–1715 (2020). https://doi.org/10.1007/s13346-020-00813-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00813-5