Abstract
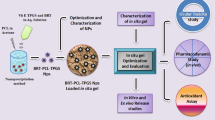
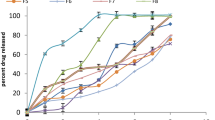
The present research work summarises the development of an in situ gelling ophthalmic nanoemulsion of brinzolamide providing sustained release and prolonged therapeutic effect for the treatment of glaucoma. Nanoemulsions were prepared using castor oil, polyoxyl 35 castor oil and polysorbate 80 and with gellan gum as the in situ gelling agent. Formulations were screened based on globule size, Zeta potential, in vitro drug release and stability towards phase separation and sol to gel conversion upon storage. Selected formulations exhibiting a low mean globule diameter (< 160 nm), narrow size distribution (polydispersity index < 0.3), quick in vitro gelling time (< 15 s) and stability for at least 6 months at 25 °C/40% RH and 40 °C/25% RH were evaluated for intraocular pressure (IOP)-lowering efficacy studies using glaucomatous rabbits. Tested nanoemulsion formulations were well tolerated and significantly decreased IOP relative to saline and placebo controls (p < 0.005). Furthermore, an appreciable increase in the area under change in IOP from baseline (ΔIOP) vs. time curve and a longer mean residence time (MRT) was also observed for the test formulations compared with commercially available suspension of brinzolamide (Azopt, Alcon Laboratories, USA). Thus, nanoemulsion formulations containing in situ gelling polymer may serve as improved drug delivery system providing superior therapeutic efficacy and better patient compliance for the treatment of glaucoma.

.
Graphical abstract



Similar content being viewed by others
References
Sall K. The efficacy and safety of brinzolamide 1% ophthalmic suspension (Azopt) as a primary therapy in patients with open-angle glaucoma or ocular hypertension. Surv Ophthalmol 2000;44(2):S155–S162.
Silver LH. Dose-response evaluation of the ocular hypotensive effect of brinzolamide ophthalmic suspension (Azopt). Surv Ophthalmol 2000;44(2):S147–S153.
Silver LH. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: results from two multicenter comfort studies. Surv Ophthalmol. 2000;44(2):S141–5.
Desantis L. Preclinical overview of brinzolamide. Surv Ophthalmol. 2000;44(2):S119–29.
Lester M. Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol. 2008;2(3):517–23.
Wu W, Li J, Wang B, Wang Z, Xu Q, Xin H. Ophthalmic delivery of brinzolamide by liquid crystalline nanoparticles: in-vitro and in-vivo evaluation. AAPS PharmSciTech. 2013;14(3):1063–71. https://doi.org/10.1208/s12249-013-9997-2.
Tuomela A, Liu P, Puranen J, Ronkko S, Laaksonen T, Kalesnykas G, et al. Brinzolamide nanocrytsal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in-vivo. Int J Pharm. 2014;467:34–41. https://doi.org/10.1016/j.ijpharm.2014.03.048.
Zhang Y, Ren K, He Z, Li H, Chen T, Lei Y et al. Development of inclusion complex of brinzolamide with hydroxypropyl-β-cyclodextrin. Carbohydr Polym. 2013;98(1):638–643; https://doi.org/10.1016/j.carbpol.2013.06. 052.
Salama H, Ghorab M, Mahmoud A, Hady M. Brinzolamide loaded-polymeric nanoparticles. Curr Sci Int 2016;05(02):147–151.
Gupta R, Tejal G, Suhagia BN. Design and development of ophthalmic nanoemulsion formulation for reducing ocular hypertension. World J Pharm Pharm Sci. 2017;6(7):695–710.
Mahboobian MM, Foroutan SM, Aboofazeli R. Brinzolamide-loaded nanoemulsions: in-vitro release evaluation. Iran J Pharm Sci 2016;12(3):75–93.
Mahboobian MM, Seyfoddin A, Aboofazeli R, Foroutan SM, Rupenthal ID. Brinzolamide-loaded nanoemulsions: ex vivo transcorneal permeation, cell viability and ocular irritation tests. Pharm Dev Technol. 2018:1–23. https://doi.org/10.1080/10837450.2018.1547748.
Sun J, Zhou Z. A novel ocular delivery of brinzolamide based on gellan gum: in-vitro and in-vivo evaluation. Drug Design, Dev Ther. 2018;12:383–9. https://doi.org/10.2147/DDDT.S153405.
Jing L, Li H, Lu L, Cai C, Xin H, Liu W. Design and evaluation of brinzolamide drug-resin in-situ thermosensitive gelling system for sustained ophthalmic drug delivery. Chem Pharm Bull 2017;62(10):1000–1008.
Araujo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nano medicines for ocular NSAIDs: safety on drug delivery. Nanomedicine. 2009;5:394–401. https://doi.org/10.1016/j.nano.2009.02.003.
Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010;141:265–76. https://doi.org/10.1016/j.jconrel.2009.10.032.
Gan L, Wang J, Jiang M, Bartlett H, Ouyang H, Eperjesi F, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov Today. 2013;18(5/6):1–8.
Tayel SA, EI-Nabarawi MA, Tadros MI. Promising ion-sensitive in-situ ocular nanoemulsion gels of terbinafine hydrochloride: design, in-vitro characterization and in-vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int J Pharm. 2013;443(1–2):293–305. https://doi.org/10.1016/j.ijpharm.2012.12.049.
Robinson G, Manning CE, Morris ER. Conformation and physical properties of the bacterial polysaccharides gellan, welan and rhamsan. In: Dickinson E, editor. Food polymers, gels, and colloids. London: Royal Society Chemistry; 1991. https://doi.org/10.1016/j.ijpharm.2011.03.043.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10(3):808–19. https://doi.org/10.1208/s12249-009-9268-4.
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NJ, et al. Nanoemulsion: concepts, development and application in drug delivery. J Control Release. 2017;252:28–49. https://doi.org/10.1016/j.jconrel.2017.03.008.
Keck CM, Muller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62:3–16. https://doi.org/10.1016/j.ejpb.2005.05.009.
Khatun R, Islam S. Development and validation of analytical method for simultaneous estimation of brinzolamide and timolol by HPLC from ophthalmic preparation. Int J Pharm Sci Res. 2014;5(3):1001–7.
Agrawal V, Desai S, Jani G. Development of RP-HPLC Method for simulataneous determination of brimonidine tartrate and brinzolamide by QbD approach and its validation. Eurasian J Anal Chem. 2016;11(2):63–78.
Mashru R, Senta B. Development and validation of spectrophotometric method for simultaneous estimation of brinzolamide and brimonidine tartrate. Asian J Pharm Life Sci. 2014;4(2):16–20.
Manoharan G, Bratty M. Development and validation of ultra violet spectrophotometric and reversed-phase high performance liquid chromatography techniques for simultaneous estimation of brinzolamide and brimonidine tartrate in ophthalmic suspension formulation. Orient J Chem. 2016;32(20):1111–20.
ICH. International Conference on Harmonization (ICH) of technical requirements for registration of pharmaceuticals for human use. Harmonized triplicate guideline on stability testing of new drug substances and products Q1A (R2), approved for adoption at step 4 of ICH process on February 2003 by ICH steering committee. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2_Guideline.pdf. Accessed July 2019.
OECD. Guideline for testing of chemical, acute eye irritation/corrosion 405: 2017. https://www.oecd.org/env/test-no-405-acute-eye-irritation-corrosion-9789264185333-en.htm. Accessed July 2019.
Chee P, Hamasaki DI. The basis of chymotrypsin-induced glaucoma. Arch Ophthalmol. 1971;85:103–6.
Percicot C, Schnell C, Debon C, Hariton C. Continuous intraocular pressure measurement by telemetry in alpha-chymotrypsin-induced glaucoma model in the rabbit: effect of timolol, dorzolamide and epinephrine. J Pharmacol Toxicol Methods. 1996;36:223–8.
Fernandez DR, Ramirez JM, Trivino A, Sanchez D, Paraiso P, Garcia D. Experimental glaucoma significantly decreases atrial natriuretic factor (ANF) receptors in the ciliary processes of the rabbit eye. Exp Eye Res. 1991;53:591–6.
Himber J, De Burlet G, Andermann G. Effects of adrenergic agents on alpha-chymotrypsin induced ocular hypertension in albino and pigmented rabbits: a comparative study. J Ocul Pharmacol. 1989;5:93–8.
Bhagav P, Upadhay H, Chandran S. Brimonidine tartrate–eudragit long-acting nanoparticles: formulation, optimization, in-vitro and in-vivo evaluation. AAPS Pharm Sci Tech. 2011;12(4):1081–101. https://doi.org/10.1208/s12249-011-9675-1.
Bhagav P, Trivedi V, Shah D, Chandran S. Sustained release ocular inserts of brimonidine tartrate for better treatment in open-angle glaucoma. Drug Deliv Trans Res. 2011;1(2):161–74. https://doi.org/10.1007/s13346-011-0018-2.
Terry JE, Hill RM. Human tear osmotic pressure: diurnal variations and the closed eye. Arch Ophthalmol. 1978;96:120–2.
Miller D. Measurement of the surface tension of tears. Arch Ophthalmol. 1969;82:368–71.
Haβe A, Keipert S. Development and characterization of microemulsions for ocular application. Eur J Pharm Biopharm. 1997;43:179–83.
United States Pharmacopeia (USP). 2nd edition., United States Pharmacopoeial Convention Inc., Rockvile, USA, 2019, Monograph <771>, 6918–6923.
Mandal A, Dhanajay P, Agrahari V, Trinh H, Joseph M, Mitra A. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67–95. https://doi.org/10.1016/j.addr.2018.01.008.
Edman P. Biopharmaceutics of ocular drug delivery. Boca Raton, FL: CRC Press; 1993.
Van Ooteghem MM. Formulations of ophthalmic solutions and suspensions. Problems and advantages. In: Edman P, editor. Biopharmaceutics of ocular drug delivery. Boca Raton: CRC; 1993. p. 31–2.
Funding
The authors would like to thank Lupin Ltd (Research Park), Pune, for providing financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhalerao, H., Koteshwara, K. & Chandran, S. Design, optimisation and evaluation of in situ gelling nanoemulsion formulations of brinzolamide. Drug Deliv. and Transl. Res. 10, 529–547 (2020). https://doi.org/10.1007/s13346-019-00697-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00697-0