Abstract
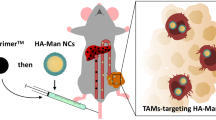
Galactomannan (GM), a natural polymer, is recognized to specifically target macrophage mannose receptors (CD206). Interestingly, some reports indicate that GM has an ability to induce pro-inflammatory (M1-like, tumericidal) polarization in macrophages, suggesting its potential use as an anti-cancer agent. Hydrazinocurcumin (HC), a pyrazole derivative of curcumin, is reported to possess increased anti-cancer efficacy over curcumin. Moreover, HC-encapsulated nanoparticles (NPs) have been reported to re-polarize tumor-associated macrophages (TAMs) from anti-inflammatory (M2-like, tumor-promoting) to pro-inflammatory phenotype. To club the therapeutic properties of both GM and HC, we synthesized self-assembled amphiphilic PEGylated GM NPs loaded with HC (PSGM-HCNPs) and evaluated their potential to re-polarize TAMs towards M1-like phenotype. PSGM-HCNPs re-polarized IL-4 polarized RAW 264.7 cells via a phenotypic switch from M2- to M1-like by elevating ROS level, decreasing CD206 and arginase-1 expressions and increasing pro-inflammatory cytokines’ secretion. Conditioned medium (CM) taken from re-polarized RAW 264.7 cells containing residual PSGM-HCNPs elevated ROS, arrested cell cycle, and induced apoptosis in 4T1, breast cancer cells, and Ehrlich’s ascites carcinoma (EAC) cells. Decreased levels of MMP-2, MMP-9, and Bcl-2 with increased levels of Bax in both 4T1 and EAC cells indicated anti-metastatic and apoptosis-inducing potential of the CM. Treatment of PSGM-HCNPs in EAC-bearing mice reduced tumor burden, increased their survival time, decreased CD206+F4/80+ cells, and increased TNF-α+F4/80+ cells signifying decrease in M2- and increase in M1-like skewness among ascitic TAMs.
















Similar content being viewed by others
References
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99.
Hofmeister V, Schrama D, Becker JC. Anti-cancer therapies targeting the tumor stroma. Cancer Immunol Immunother. 2008;57(1):1–17.
Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176(4):1564–76.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–7.
Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8.
Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–84.
Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–44.
Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–26.
Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–27.
Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40(11):1660–7.
Weigert A, Sekar D, Brüne B. Tumor-associated macrophages as targets for tumor immunotherapy. Immunotherapy. 2009;1(1):83–95.
Arinaga S, Karimine N, Takamuku K, Nanbara S, Nagamatsu M, Ueo H, et al. Enhanced production of interleukin 1 and tumor necrosis factor by peripheral monocytes after lentinan administration in patients with gastric carcinoma. Int J Immunopharmacol. 1992;14(1):43–7.
Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang K-H, et al. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999;41(2):157–64.
Moretão MP, Zampronio AR, Gorin PA, Iacomini M, Oliveira MBM. Induction of secretory and tumoricidal activities in peritoneal macrophages activated by an acidic heteropolysaccharide (ARAGAL) from the gum of Anadenanthera colubrina (Angico branco). Immunol Lett. 2004;93(2–3):189–97.
Moretton MA, Chiappetta DA, Andrade F, Das Neves J, Ferreira D, Sarmento B, et al. Hydrolyzed galactomannan-modified nanoparticles and flower-like polymeric micelles for the active targeting of rifampicin to macrophages. J Biomed Nanotechnol. 2013;9(6):1076–87.
Duncan CJ, Pugh N, Pasco DS, Ross SA. Chemistry f. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. J Agric Food Chem. 2002;50(20):5683–5.
Y-m H, Chun S-H, S-t H, Y-c K, H-d C, Lee K-w. Immune enhancing effect of a Maillard-type lysozyme-galactomannan conjugate via signaling pathways. Int J Biol Macromol. 2013;60:399–404.
Noleto GR, Mercê ALR, Iacomini M, Gorin PA, Soccol VT, Oliveira MBM. Effects of a lichen galactomannan and its vanadyl (IV) complex on peritoneal macrophages and leishmanicidal activity. Mol Cell Biochem. 2002;233(1–2):73–83.
Rathore R, Jain JP, Srivastava A, Jachak S, Kumar N. analysis b. Simultaneous determination of hydrazinocurcumin and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharm Biomed Anal. 2008;46(2):374–80.
Wang X, Zhang Y, Zhang X, Tian W, Feng W, Chen T. The curcumin analogue hydrazinocurcumin exhibits potent suppressive activity on carcinogenicity of breast cancer cells via STAT3 inhibition. Int J Oncol. 2012;40(4):1189–95.
Zhang X, Tian W, Cai X, Wang X, Dang W, Tang H, et al. Hydrazinocurcumin encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS One. 2013;8(6):e65896.
Wang Y, Lin Y-X, Qiao S-L, An H-W, Ma Y, Qiao Z-Y, et al. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–63.
Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–94.
Bansal R, Singh A, Gandhi R, Pant A, Kumar P, Gupta K. Galactomannan-PEI based non-viral vectors for targeted delivery of plasmid to macrophages and hepatocytes. Eur J Pharm Biopharm. 2014;87(3):461–71.
Coviello T, Alhaique F, Dorigo A, Matricardi P, Grassi M. Two galactomannans and scleroglucan as matrices for drug delivery: preparation and release studies. Eur J Pharm Biopharm. 2007;66(2):200–9.
Nurnadiah R, Kamarun D, Li AR, Ahmad MR. Synthesis and characterization of crosslinked galactomannan nanoparticles for drug delivery application. Adv Mater Res. 2013;812:12–9.
Dangaj D, Abbott KL, Mookerjee A, Zhao A, Kirby PS, Sandaltzopoulos R, et al. Mannose receptor (MR) engagement by mesothelin GPI anchor polarizes tumor-associated macrophages and is blocked by anti-MR human recombinant antibody. PLoS One. 2011;6(12):e28386.
Binnemars-Postma K, Storm G, Prakash J. Nanomedicine strategies to target tumor-associated macrophages. Int J Mol Sci. 2017;18(5):979.
Okalebo FA, Ngaruiya MN, Changwony P, Oluka MO, Karume DW, Maloba KN. The antinociceptive effects of Hydrazinocurcumin. Afr J Pharm Pharmacol Ther. 2013;2(2):66–9.
Crescenzi V, Dentini M, Risica D, Spadoni S, Skjåk-Bræk G, Capitani D, et al. C (6)-oxidation followed by C (5)-epimerization of guar gum studied by high field NMR. Biomacromolecules. 2004;5(2):537–46.
Kumari M, Ray L, Purohit M, Patnaik S, Pant A, Shukla Y, et al. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. Eur J Pharm Biopharm. 2017;117:346–62.
Lombardo D, Kiselev MA, Magazù S, Calandra P. Amphiphiles self-assembly: basic concepts and future perspectives of supramolecular approaches. Adv Cond Matter Phys. 2015;2015.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Part 1):6387–92.
Jin Y, Ma X, Feng S, Liang X, Dai Z, Tian J, et al. Hyaluronic acid modified tantalum oxide nanoparticles conjugating doxorubicin for targeted cancer theranostics. Bioconjug Chem. 2015;26(12):2530–41.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55.
Liu C-Y, Xu J-Y, Shi X-Y, Huang W, Ruan T-Y, Xie P, et al. M2-polarized tumor-associated macrophages promoted epithelial–mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Investig. 2013;93(7):844–54.
Shin K-S. The chemical characteristics and immune-modulating activity of polysaccharides isolated from cold-brew coffee. Prev Nutr Food Sci. 2017;22(2):100–6.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Tan H-Y, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxidative Med Cell Longev. 2016;2016:1–16.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491.
Gong D, Shi W, Yi S-j, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13(1):13–31.
Haak-Frendscho M, Wynn T, Czuprynski C, Paulnock D. Transforming growth factor-β1 inhibits activation of macrophage cell line RAW 264.7 for cell killing. Clin Exp Immunol. 1990;82(2):404–10.
Kaushik NK, Kaushik N, Min B, Choi KH, Hong YJ, Miller V, et al. Cytotoxic macrophage-released tumour necrosis factor-alpha (TNF-α) as a killing mechanism for cancer cell death after cold plasma activation. J Phys D Appl Phys. 2016;49(8):084001.
Kim J, Lee S, Park J, Yoo Y. TNF-α-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X L. Cell Death Differ. 2010;17(9):1420–34.
Shih S-C, Stutman O. Cell cycle-dependent tumor necrosis factor apoptosis. Cancer Res. 1996;56(7):1591–8.
Akutagawa N, Nishikawa A, Iwasaki M, Fujimoto T, Teramoto M, Kitajima Y, et al. Expression of vascular endothelial growth factor and E-cadherin in human ovarian cancer: association with ascites fluid accumulation and peritoneal dissemination in mouse ascites model. Jpn J Cancer Res. 2002;93(6):644–51.
Kalish SV, Lyamina SV, Usanova EA, Manukhina EB, Larionov NP, Malyshev IY. Macrophages reprogrammed in vitro towards the M1 phenotype and activated with LPS extend lifespan of mice with ehrlich ascites carcinoma. Med Sci Monit Basic Res. 2015;21:226–34.
Kono Y, Kawakami S, Higuchi Y, Maruyama K, Yamashita F, Hashida M. Antitumor effect of nuclear factor-κB decoy transfer by mannose-modified bubble lipoplex into macrophages in mouse malignant ascites. Cancer Sci. 2014;105(8):1049–55.
Joseph MM, Aravind S, George SK, Varghese S, Sreelekha T. A galactomannan polysaccharide from Punica granatum imparts in vitro and in vivo anticancer activity. Carbohydr Polym. 2013;98(2):1466–75.
Sone Y, Isoda-Johmura M, Misaki A. Isolation and chemical characterization of polysaccharides from Iwatake, Gyrophora esculenta Miyoshi. Biosci Biotechnol Biochem. 1996;60(2):213–5.
Georgoudaki A-M, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15(9):2000–11.
Lee C, Bae S-JS, Joo H, Bae H. Melittin suppresses tumor progression by regulating tumor-associated macrophages in a Lewis lung carcinoma mouse model. Oncotarget. 2017;8(33):54951–65.
Kratochvill F, Neale G, Haverkamp JM, Van de Velde L-A, Smith AM, Kawauchi D, et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12(11):1902–14.
Ashley JW, Hancock WD, Nelson AJ, Bone RN, Hubert MT, Wohltmann M, et al. Polarization of macrophages towards M2 phenotype is favored by reduction in iPLA2β. J Biol Chem. 2016;291(44):23268–81.
Acknowledgments
KCG thanks the Indian Council of Medical Research (ICMR), New Delhi, for the award of Dr. A.S. Paintal Distinguished Scientist Chair at CSIR-IGIB, Delhi. MK thanks CSIR for providing Senior Research Fellowship. Dr. B. Jagadeesh and Dr. M.K.R. Mudiam from CSIR-IICT, Hyderabad, are acknowledged for NMR and LC-MS studies, respectively. Ms. N. Arjaria, CSIR-IITR and Mr. Jaishankar of CSIR-IITR Lucknow are acknowledged for their help in TEM studies. Dr. P.N. Saxena of CSIR-IITR Lucknow is acknowledged for the help in SEM studies. The authors are thankful to Mr. S.H.N. Naqvi of CSIR-IITR Lucknow for helping in animal experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, M., Purohit, M.P., Pahuja, R. et al. Pro-inflammatory macrophage polarization enhances the anti-cancer efficacy of self-assembled galactomannan nanoparticles entrapped with hydrazinocurcumin. Drug Deliv. and Transl. Res. 9, 1159–1188 (2019). https://doi.org/10.1007/s13346-019-00661-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00661-y