Abstract
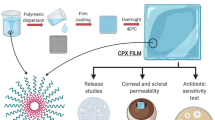
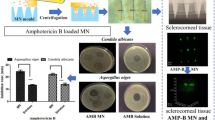
Penetration of antibiotics into and through the cornea is a major limiting factor in the treatment of ocular infections. Several strategies are in vogue to overcome this limitation such as use of fortified drops, gels, and subconjunctival injections. Here, we present the fabrication of rapidly dissolving polymeric microneedle array to effectively deliver besifloxacin through the cornea. Microneedles were prepared using polyvinyl alcohol and polyvinyl pyrrolidone by the micromolding technique. The model fluoroquinolone antibiotic, besifloxacin, was loaded in 36 microneedles arranged in a 6 × 6 array format within a 1 cm2 area. The average height and base width of microneedles was 961 ± 27 and 366 ± 16 μm, respectively. Each microneedle array contained 103.4 ± 8.5 μg of besifloxacin. Cryosectioning and confocal microscopy of excised human cornea revealed that microneedles penetrated to a depth of up to 200 μm. Microneedles were found to completely dissolve in the cornea within 5 min. Application of microneedles for 5 min significantly (p < 0.05) improved the besifloxacin deposition and permeation through the cornea compared with free besifloxacin solution. Similarly, besifloxacin-loaded microneedles showed greater antibacterial activity in Staphylococcus aureus-infected cornea in comparison to free besifloxacin solution. Taken together, rapidly dissolving microneedles can be developed to effectively deliver besifloxacin to treat bacterial infections in the cornea and eye.







Similar content being viewed by others
References
Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–21.
Whitcher J, Srinivasan M. Corneal ulceration in the developing world—a silent epidemic. Br J Ophthalmol. 1997;81(8):622–3. https://doi.org/10.1136/bjo.81.8.622.
Dandona R, Dandona L. Review of findings of the Andhra Pradesh Eye Disease Study: policy implications for eye-care services. Indian J Ophthalmol. 2001;49(4):215–34.
Kanski JJ, Bowling B. Clinical ophthalmology: a systematic approach. Elsevier Health Sciences; 2011. p. 167–233.
Donahue SP, Khoury JM, Kowalski RP. Common ocular infections: a prescriber’s guide. Drugs. 1996;52(4):526–40. https://doi.org/10.2165/00003495-199652040-00006.
Maurice D. Practical issues in intravitreal drug delivery. J Ocul Pharmacol Ther. 2001;17(4):393–401. https://doi.org/10.1089/108076801753162807.
Lee DA, Hersh P, Kersten D, Melamed S. Complications of subconjunctival 5-fluorouracil following glaucoma filtering surgery. Ophthalmic Surg Lasers Imaging Retina. 1987;18(3):187–90.
Quinn HL, Kearney M-C, Courtenay AJ, McCrudden MT, Donnelly RF. The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv. 2014;11(11):1769–80. https://doi.org/10.1517/17425247.2014.938635.
Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164–82. https://doi.org/10.1016/j.jconrel.2017.05.029.
Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68. https://doi.org/10.1016/j.addr.2012.04.005.
Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007;48(9):4038–43. https://doi.org/10.1167/iovs.07-0066.
Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26(2):395–403. https://doi.org/10.1007/s11095-008-9756-3.
Kim YC, Grossniklaus HE, Edelhauser HF, Prausnitz MR. Intrastromal delivery of bevacizumab using microneedles to treat corneal neovascularization. Invest Ophthalmol Vis Sci. 2014;55(11):7376–86. https://doi.org/10.1167/iovs.14-15257.
Song HB, Lee KJ, Seo IH, Lee JY, Lee S-M, Kim JH, et al. Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. Journal of Controlled Release. 2015;209(Supplement C):272–9. https://doi.org/10.1016/j.jconrel.2015.04.041.
US Dept of Health and Human Services. NDA 22–308. NDA approval. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/ 022308s000ltr.pdf.
Malhotra R, Ackerman S, Gearinger LS, Morris TW, Allaire C. The safety of besifloxacin ophthalmic suspension 0.6% used three times daily for 7 days in the treatment of bacterial conjunctivitis. Drugs R&D. 2013;13(4):243–52. https://doi.org/10.1007/s40268-013-0029-1.
George S, Atchison DA. The Eye. In: George S, Atchison DA, editors. The Eye and Visual Optical Instruments. Cambridge, UK: Cambridge University Press; 1997. p. 291–316
Zaman NT, Yang Y-Y, Ying JY. Stimuli-responsive polymers for the targeted delivery of paclitaxel to hepatocytes. Nano Today. 2010;5(1):9–14. https://doi.org/10.1016/j.nantod.2009.12.008.
Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60. https://doi.org/10.1208/s12248-010-9183-3.
Duxfield L, Sultana R, Wang R, Englebretsen V, Deo S, Rupenthal ID, et al. Ocular delivery systems for topical application of anti-infective agents. Drug Dev Ind Pharm. 2016;42(1):1–11. https://doi.org/10.3109/03639045.2015.1070171.
Neena Washington CW, Clive Wils. Physiological Pharmaceutics: Barriers to Drug Absorption. Second ed.: CRC Press, Taylor & Francis group; 2001. p. 249-69.
Schoenwald RD. Ocular drug delivery. Clin Pharmacokinet. 1990;18(4):255–69. https://doi.org/10.2165/00003088-199018040-00001.
Thakur Singh RR, Tekko I, McAvoy K, McMillan H, Jones D, Donnelly RF. Minimally invasive microneedles for ocular drug delivery. Expert Opin Drug Deliv. 2017;14(4):525–37. https://doi.org/10.1080/17425247.2016.1218460.
Thakur RRS, Tekko IA, Al-Shammari F, Ali AA, McCarthy H, Donnelly RF. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv Transl Res. 2016;6(6):800–15. https://doi.org/10.1007/s13346-016-0332-9.
Tamboli V, Mishra GP, Mitra AK. Biodegradable polymers for ocular drug delivery. Adv Ocul Drug Deliv. 2012;2012:65–86.
Yang S, Feng Y, Zhang L, Chen N, Yuan W, Jin T. A scalable fabrication process of polymer microneedles. Int J Nanomedicine. 2012;7:1415–22. https://doi.org/10.2147/IJN.S28511.
Bhatnagar S, Chawla SR, Kulkarni OP, Venuganti VVK. Zein microneedles for transcutaneous vaccine delivery: fabrication, characterization, and in vivo evaluation using ovalbumin as the model antigen. ACS Omega. 2017;2(4):1321–32. https://doi.org/10.1021/acsomega.7b00343.
Bediz B, Korkmaz E, Khilwani R, Donahue C, Erdos G, Falo LD Jr, et al. Dissolvable microneedle arrays for intradermal delivery of biologics: fabrication and application. Pharm Res. 2014;31(1):117–35. https://doi.org/10.1007/s11095-013-1137-x.
Lee IC, He J-S, Tsai M-T, Lin K-C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J Mater Chem B. 2015;3(2):276–85. https://doi.org/10.1039/c4tb01555j.
Acknowledgements
This work was financially supported by the Indian Council of Medical Research (ICMR, IRIS ID: 2015-00100). Texture analyzer was procured using a grant from the Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure (DST-FIST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 590 kb)
Rights and permissions
About this article
Cite this article
Bhatnagar, S., Saju, A., Cheerla, K.D. et al. Corneal delivery of besifloxacin using rapidly dissolving polymeric microneedles. Drug Deliv. and Transl. Res. 8, 473–483 (2018). https://doi.org/10.1007/s13346-017-0470-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0470-8