Abstract
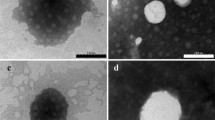
While eye drops account for the majority of ophthalmic formulation for drug delivery, their efficiency is limited by rapid pre-corneal loss. In this study, we investigate nanogel suspensions in order to improve the topical ocular therapy by reducing dosage and frequency of administration. We synthesized self-assembling nanogels of 140 nm by grafting side chains of poly(N-tert-butylacrylamide) (PNtBAm) on methylcellulose via cerium ammonium nitrate. Successful grafting of PNtBAm onto methylcellulose (MC) was confirmed by both NMR and ATR. Synthesized molecules (MC-g-PNtBAm) self-assembled in water driven by hydrophobic interaction of the grafted side chains creating colloid solutions. Materials were synthesized by changing feed ratios of acid, initiator and monomer in order to control the degree of hydrophobic modification. The nanogels were tested for different degrees of grafting. Viability studies performed with HCE cells testified to the biocompatibility of poly(N-tert-butylacrylamide) grafted methylcellulose nanogels. Dexamethasone was entrapped with an efficiency superior to 95 % and its release presented minimal burst phase. Diffusion of drug from the nanogels was found to be delayed by increasing the degree of grafting. The release profile of the entrapped compound from the MC-g-PNtBAm nanogels can thus be tuned by simply adjusting the degree of hydrophobic modification. MC-g-PNtBAm nanogels present promising properties for ocular drug delivery.









Similar content being viewed by others
References
Jansook P, Stefánsson E, Thorsteinsdóttir M, Sigurdsson BB, Kristjánsdóttir SS, Bas JF, et al. Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: formulation of dorzolamide eye drop microparticle suspension. Eur J Pharm Biopharm. 2010;76(2):208–14.
Loftsson T, Hreinsdóttir D, Stefánsson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J Pharm Pharmacol. 2007;59(5):629–35.
Diebold Y, Calonge M. Progress in retinal and eye research applications of nanoparticles in ophthalmology. Prog Retin Eye Res. 2010;29(6):596–609.
Gaudana R, Jwala J, Boddu SHS, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197–216.
Bozdag S, Weyenberg W, Adriaens E, Dhondt MMM, Vergote V, Vervaet C, et al. In vitro evaluation of gentamicin- and vancomycin-containing minitablets as a replacement for fortified eye drops. Drug Dev Ind Pharm. 2010;36(11):1259–70.
Li X, Zhang Z, Chen H. Development and evaluation of fast forming nano-composite hydrogel for ocular delivery of diclofenac. Int J Pharm. 2013;448(1):96–100.
Patton TF, Robinson JR. Quantitative precorneal disposition of topically applied pilocarpine nitrate in rabbit eyes. J Pharm Sci. 1976;65(9):1295–301.
Qi H, Chen W, Huang C, Li L, Chen C, Li W, et al. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm. 2007;337(1–2):178–87.
Urtti A, Pipkin JD, Rork G, Sendo T, Finne U, Repta AJ. Controlled drug delivery devices for experimental ocular studies with timolol 2. Ocular and systemic absorption in rabbits. Int J Pharm. 1990;61(3):241–9.
Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136(1):2–13.
Sosnik A, das Neves J, Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog Polym Sci. 2014;39(12):2030–75.
Gan L, Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov Today. 2013;18(5–6):290–7.
Li N, Zhuang C, Wang M, Sun X, Nie S, Pan W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm. 2009;379(1):131–8.
Liu R, Sun L, Fang S, Wang S, Chen J, Xiao X, et al. Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: development, characterization, in vitro permeation and in vivo pharmacokinetic studies. Informa Healthcare USA. 2015. doi:10.3109/10837450.2015.1026607.
Abd El-Rehim HA, Swilem AE, Klingner A, Hegazy E-SA, Hamed AA. Developing the potential ophthalmic applications of pilocarpine entrapped into polyvinylpyrrolidone-poly(acrylic acid) nanogel dispersions prepared by γ radiation. Biomacromolecules. 2013;14(3):688–98.
Liu S, Jones L, Gu FX. Nanomaterials for ocular drug delivery. Macromol Biosci. 2012;12(5):608–20.
Moya-Ortega MD, Alvarez-Lorenzo C, Concheiro A, Loftsson T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int J Pharm. 2012;428(1–2):152–63.
Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog Polym Sci. 2008;33(4):448–77.
Samah NA, Williams N, Heard CM. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int J Pharm. 2010;401(1–2):72–8.
Sawada SI, Sasaki Y, Nomura Y, Akiyoshi K. Cyclodextrin-responsive nanogel as an artificial chaperone for horseradish peroxidase. Colloid Polym Sci. 2011;289(5–6):685–91.
Guo Q, Wu Z, Zhang X, Sun L, Li C. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH. Soft Matter. 2014;10(6):911.
Kettel MJ, Dierkes F, Schaefer K, Moeller M, Pich A. Aqueous nanogels modified with cyclodextrin. Polymer. 2011;52(9):1917–24.
Rafie F, Javadzadeh Y, Javadzadeh AR, Ghavidel LA, Jafari B, Moogooee M, et al. In vivo evaluation of novel nanoparticles containing dexamethasone for ocular drug delivery on rabbit eye. Curr Eye Res. 2010;35(12):1081–9.
Li X, Zhang Z, Li J, Sun S, Weng Y, Chen H. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale. 2012;4(15):4667.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J Drug Target. 2011;19(6):409–17.
Ganguly K, Chaturvedi K, More UA, Nadagouda MN, Aminabhavi TM. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J Control Release. 2014;193:162–73.
Moya-Ortega MD, Alves TFG, Alvarez-Lorenzo C, Concheiro A, Stefánsson E, Thorsteinsdóttir M, et al. Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int J Pharm. 2013;441(1–2):507–15.
Nagarwal RC, Singh PN, Kant S, Maiti P, Pandit JK. Chitosan nanoparticles of 5-fluorouracil for ophthalmic delivery: characterization, in-vitro and in-vivo study. Chem Pharm Bull. 2011;59(2):272–8.
Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224(1–2):19–38.
Lemarchand C, Gref R, Couvreur P. Polysaccharide-decorated nanoparticles. Eur J Pharm Biopharm. 2004;58(2):327–41.
Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Wan Kim S, et al. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Release. 1998;54(3):313–20.
Akiyoshi K, Sunamoto J. Supramolecular assembly of hydrophobized polysaccharides. Supramol Sci. 1996;3(1–3):157–63.
Sawada S-I, Nomura Y, Aoyama Y, Akiyoshi K. Heat shock protein-like activity of a nanogel artificial chaperone for citrate synthase. J Bioact Compat Polym. 2006;21(6):487–501.
Gu X, Schmitt M, Hiasa A, Nagata Y, Ikeda H, Sasaki Y, et al. A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res. 1998;58:3385–90.
Yoncheva K, Vandervoort J, Ludwig A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm Dev Technol. 2011;16(1):29–35.
Stella B, Arpicco S, Rocco F, Burgalassi S, Nicosia N, Tampucci S, et al. Nonpolymeric nanoassemblies for ocular administration of acyclovir: pharmacokinetic evaluation in rabbits. Eur J Pharm Biopharm. 2012;80(1):39–45.
Arisz PW, Kauw HJJ, Boon JJ. Substituent distribution along the cellulose backbone in O-methylcelluloses using GC and FAB-MS for monomer and oligomer analysis. Carbohydr Res. 1995;271(1):1–14.
Bhowmik M, Bain MK, Ghosh LK, Chattopadhyay D. Effect of salts on gelation and drug release profiles of methylcellulose-based ophthalmic thermo-reversible in situ gels. Pharm Dev Technol. 2011;16(4):385–91.
Li N, Yu M, Deng L, Yang J, Dong A. Thermosensitive hydrogel of hydrophobically-modified methylcellulose for intravaginal drug delivery. J Mater Sci Mater Med. 2012;23(8):1913–9.
Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–9.
Ngadaonye JI, Cloonan MO, Geever LM, Higginbotham CL. Synthesis and characterisation of thermo-sensitive terpolymer hydrogels for drug delivery applications. J Polym Res. 2011;18(6):2307–24.
Liu HY, Zhu XX. Lower critical solution temperatures of N-substituted acrylamide copolymers in aqueous solutions. Polymer. 1999;40(25):6985–90.
Moran MT, Carroll WM, Selezneva I, Gorelov A, Rochev Y. Cell growth and detachment from protein-coated PNIPAAm-based copolymers. J Biomed Mater Res. 2007.
Yldz B, Işk B, Kş M. Synthesis and characterization of thermoresponsive isopropylacrylamide-acrylamide hydrogels. Eur Polym J. 2002;38(7):1343–7.
Musial W, Voncina B, Pluta J, Kokol V. The study of release of chlorhexidine from preparations with modified thermosensitive poly-N-isopropylacrylamide microspheres. ScientificWorld Journal. 2012. doi:10.1100/2012/243707.
Muramatsu K, Wada T, Hirai H, Miyawaki FSY. Poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)- grafted hyaluronan as an injectable and self-assembling scaffold for cartilage tissue engineering. J Biomed Sci Eng. 2012;5:639–46.
Hoffman AS, Stayton PS, Bulmus V, Chen G, Chen J, Cheung C, et al. Really smart bioconjugates of smart polymers and receptor proteins. J Biomed Mater Res. 2000;52(4):577–86.
Pourjavadi A, Mahdavinia GR, Omidian H. Modified chitosan. I. Optimized cerium ammonium nitrate-induced synthesis of chitosan-graft-polyacrylonitrile. J of Appl Poly Sci. 2003;88:2048–54.
Zhu Z, Zhuo R. Controlled release of carboxylic-containing herbicides by starch-g-poly (butyl acrylate). J of Appl Poly Sci. 2001;81:1535–43.
Fanta GF, Burr RC, Doane WM. Polymerization of alkyl acrylates and alkyl methacrylates with starch. J of Appl Poly Sci. 1980;25:2285–94.
Mcdowall DJ, Gupta BS, Stannett VT. Grafting cellulose of vinyl monomers to cellulose by ceric initiation. Prog Polym Sci. 1984;10:1–50.
Pulat M, Isakoca C. Chemically induced graft copolymerization of vinyl monomers onto cotton fibers. J Appl Polym Sci. 2006;100(3):2343–7.
Zahran MK. Grafting of Methacrylic acid and other vinyl monomers onto cotton fabric using Ce (IV) ion–cellulose thiocarbonate redox system. J Polym Res. 2005;13(1):65–71.
Kaizerman S, Mino G, Meinhold LF. The polymerization of vinyl monomers in cellulosic fibers. Text Res J. 1962;32(2):136–40.
Hebeish A, Kaktouch A, El-Rafie MH. Graft copolymerization of vinyl monomers with modified cotton. II. Grafting of acrylonitrile and methyl methacrylate on acetylated cotton. J of Appl Poly Sci. 1971;15:11–24.
Gu L, Gu G, Saadet O. Graft copolymerization of acrylic acid onto cellulose: effects of pretreatments and crosslinking agent. J of Appl Poly Sci. 2000;80:2267–72.
Kubota H, Shiobara N. Photografting of N-isopropylacrylamide on cellulose and temperature-responsive character of the resulting grafted celluloses. React & Funct Poly. 1998;37:219–24.
Chatterjee S, Sarkar S, Bhattacharyya SN. Colloidal ferric oxide: a new photosensitizer for grafting acrylamide onto cellulose acetate films. Polymer. 1993;34(9):1979–80.
Chiriac AP, Neamtu I, Cazacu G, Simionescu CI. An investigation of the grafting of cellulose powder with acrylamide under a magnetic field. Die Angewandte Makromolekulare Chemie. 1997;246(4224):1–9.
Gupta KC, Khandekar K. Temperature-responsive cellulose by ceric(IV) ion-initiated graft copolymerization of N-isopropylacrylamide. Biomacromolecules. 2003;4(3):758–65.
Ahn HR, Tak TM. Optimization grafting conditions and characterization of methyl cellulose grafted polyacrylonitrile copolymer. Macromol Res. 2014;22(3):318–23.
Hebeish A, Mehta PC. Cerium-initiated grafting of acrylonitrile onto cellulosic materials. J Appl Poly Sci. 1968;12:1625–47.
Okieimen FE, Ogbeifun DE. Graft copolymerization of modified cellulose, grafting of acrylonitrile, and methyl methacrylate on carboxy methyl cellulose. J Appl Polym Sci. 1996;59(6):981–6.
Eromosele IC, Eromosele CO, Zanna HK. Graft copolymerization of acrylic acid on methylcellulose by ceric ion/p-xylene redox pair. J Appl Polym Sci. 2002;84(3):500–4.
Griffith M. Functional human corneal equivalents constructed from cell lines. Science. 1999;286(5447):2169–72.
Akiyoshi K, Deguchi S, Tajima H, Nishikawa T, Sunamoto J. Microscopic structure and thermoresponsiveness of a hydrogel nanoparticle by self-assembly of a hydrophobized polysaccharide. Macromolecules. 1997;9297(96):857–61.
Verma MS, Liu S, Chen YY, Meerasa A, Gu FX. Size-tunable nanoparticles composed of dextran-b-poly(D,L-lactide) for drug delivery applications. Nano Res. 2012;5(1):49–61.
Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VHL. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res. 2004;21(4):641–8.
Funami T, Kataoka Y, Hiroe M, Asai I, Takahashi R, Nishinari K. Thermal aggregation of methylcellulose with different molecular weights. Food Hydrocoll. 2007;21(1):46–58.
Akiyoshi K, Deguchi S, Moriguchi N, Yamaguchi S, Sunamoto J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules. 1993;26(12):3062–8.
Saldías C, Velásquez L, Quezada C, Leiva A. Physicochemical assessment of dextran-g-poly (ɛ-caprolactone) micellar nanoaggregates as drug nanocarriers. Carbohydr Polym. 2015;117:458–67.
Guo Y, Zhang L, Li H, Han Y, Zhou J, Wang X. Self-assembly and paclitaxel loading capacity of α-tocopherol succinate-conjugated hydroxyethyl cellulose nanomicelle. Colloid Polym Sci. 2016;294:135–43.
Calejo MT, Kjøniksen AL, Maleki A, Nyström B, Sande SA. Microparticles based on hydrophobically modified chitosan as drug carriers. J Appl Polym Sci. 2014;131(7):1–11.
Vafaei SY, Esmaeili M, Amini M, Atyabi F, Ostad SN, Dinarvand R. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr Polym. 2016;144:371–81.
Yang Y, Guo Y, Sun R, Wang X. Self-assembly and β-carotene loading capacity of hydroxyethyl cellulose-graft-linoleic acid nanomicelles. Carbohydr Polym. 2016;145:56–63.
Wang M, Huang M, Wang J, Ye M, Deng Y, Li H, et al. Facile one-pot synthesis of self-assembled folate-biotin-pullulan nanoparticles for targeted intracellular anticancer drug delivery. J Nanomater. 2016. doi:10.1155/2016/5752921.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The funding support of the Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged.
Affiliations
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
The authors certify that appropriate ethical approval was obtained where warranted.
Informed consent
The authors certify that where appropriate informed consent was obtained.
Rights and permissions
About this article
Cite this article
Jamard, M., Hoare, T. & Sheardown, H. Nanogels of methylcellulose hydrophobized with N-tert-butylacrylamide for ocular drug delivery. Drug Deliv. and Transl. Res. 6, 648–659 (2016). https://doi.org/10.1007/s13346-016-0337-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0337-4