Abstract
We describe, for the first time, considerations in the sterile manufacture of polymeric microneedle arrays. Microneedles (MN) made from dissolving polymeric matrices and loaded with the model drugs ovalbumin (OVA) and ibuprofen sodium and hydrogel-forming MN composed of “super-swelling” polymers and their corresponding lyophilised wafer drug reservoirs loaded with OVA and ibuprofen sodium were prepared aseptically or sterilised using commonly employed sterilisation techniques. Moist and dry heat sterilisation, understandably, damaged all devices, leaving aseptic production and gamma sterilisation as the only viable options. No measureable bioburden was detected in any of the prepared devices, and endotoxin levels were always below the US Food & Drug Administration limits (20 endotoxin units/device). Hydrogel-forming MN were unaffected by gamma irradiation (25 kGy) in terms of their physical properties or capabilities in delivering OVA and ibuprofen sodium across excised neonatal porcine skin in vitro. However, OVA content in dissolving MN (down from approximately 101.1 % recovery to approximately 58.3 % recovery) and lyophilised wafer-type drug reservoirs (down from approximately 99.7 % recovery to approximately 60.1 % recovery) was significantly reduced by gamma irradiation, while the skin permeation profile of ibuprofen sodium from gamma-irradiated dissolving MN was markedly different from their non-irradiated counterparts. It is clear that MN poses a very low risk to human health when used appropriately, as evidenced here by low endotoxin levels and absence of microbial contamination. However, if guarantees of absolute sterility of MN products are ultimately required by regulatory authorities, it will be necessary to investigate the effect of lower gamma doses on dissolving MN loaded with active pharmaceutical ingredients and lyophilised wafers loaded with biomolecules in order to avoid the expense and inconvenience of aseptic processing.

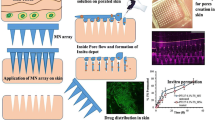
Concept map for the sterile manufacture of polymeric microneedle arrays and lyophilised wafers




Similar content being viewed by others
References
R.F. Donnelly, T.R.R. Singh, D.I.J Morrow, A.D. Woolfson. Microneedle mediated transdermal and intradermal drug delivery. Wiley Online Library (2012).
Donnelly RF, Morrow DIJ, Singh TRR, Migalska K, McCarron PA, O’Mahony C, et al. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm. 2009;35:1242–54.
Migalska K, Morrow DIJ, Garland MJ, Thakur RRS, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res. 2011;28:1919–30.
McCrudden MTC, Alkilani AZ, McCrudden CM, McAlister E, McCarthy HO, Woolfson AD, et al. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J Control Release. 2014;180:71–80.
McCrudden MTC, Torrisi BM, Al-Zahrani S, McCrudden CM, Zaric M, Scott CJ, et al. Laser-engineered dissolving microneedle arrays for protein delivery: potential for enhanced intradermal vaccination. J Pharm Pharmacol. 2014. doi:10.1111/jphp.12248.
Donnelly RF, Thakur RRS, Garland MJ, Migalska K, Majithiya R, McCrudden CM, et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22:4879–90.
Donnelly RF, Thakur RRS, McCrudden MTC, Zaid-Alkilani A, O’Mahony C, Armstrong K, et al. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: potential for enhanced patient safety. Int J Pharm. 2013;451:76–91.
The British Pharmacopoeia 2013 (incorporation of the European Pharmacopoeia). http://www.pharmacopoeia.co.uk/2013/. Accessed 6th Oct. (2013)
Donnelly RF, Majithiya R, Singh TR, Morrow DI, Garland MJ, Demir YK, et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28:41–57.
Thakur RRS, McCarron PA, Woolfson AD, Donnelly RF. Investigation of swelling and network parameters of poly (ethylene glycol)-crosslinked poly (methyl vinyl ether-co-maleic acid) hydrogels. Eur Polym J. 2009;45:1239–49.
Al-Zahrani S, Zaric M, McCrudden C, Scott C, Kissenpfennig A, Donnelly RF. Microneedle mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv. 2012;9:541–50.
Clough R, Gillen K, Malone G, Wallace J. Color formation in irradiated polymers. Radiat Phys Chem. 1996;48:583–94.
Clough R, Malone G, Gillen K, Wallace J, Sinclair M. Discoloration and subsequent recovery of optical polymers exposed to ionizing radiation. Polym Degrad Stab. 1995;49:305–13.
Wallace J, Sinclair M, Gillen K, Clough R. Color center annealing in γ-irradiated polystyrene, under vacuum and air atmospheres. Radiat Phys Chem. 1993;41:85–100.
Klemchuk PP. Protecting polymers against damage from gamma radiation. Radiat Phys Chem. 1993;41:165–72.
Gillen K, Wallace J, Clough R. Dose-rate dependence of the radiation-induced discoloration of polystyrene. Radiat Phys Chem. 1993;41:101–13.
Williams J, Dunn T, Stannett V. Mobility as a mechanism for radiation stabilization of polypropylene. Radiat Phys Chem. 1982;19:291–6.
M. Gahleitner, J. Wolfschwenger, J. Fiebig, K. Dobianer, C. Hametner. Sterilization effects on polypropylene: technology and polymer type effects. Proc. 9th European Place. Conf. (2003) 3.
King RS. Method for reducing gamma radiation sterilization induced discoloration. Off Gaz U S Patent Trademark Off Patents. 1996;1191:992.
L.K. Massey. The effect of sterilization methods on plastics and elastomers, 2nd edn. Access Online via Elsevier (2005).
Guidance for industry, pyrogen and endotoxin testing question and answers 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM310098.pdf (2014).
Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O’Mahony C, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26:2513–22.
Beisson F, Aoubala M, Marull S, Moustacas-Gardies AM, Voultoury R, Arondel V. Use of the tape stripping technique for directly quantifying esterase activities in human stratum corneum. Anal Biochem. 2001;290:179–85.
Engelrud T. Stratum corneum chymotryptic enzyme: evidence of its location in the stratum corneum intercellular space. Eur J Dermatol. 1992;2:50–5.
Silindir M, Özer AY. Sterilization methods and comparison of e-beam sterilization with gamma radiation sterilization. FABAD J Pharm Sci. 2009;34:43–53.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/K020234/1).
Conflict of interest
Ryan Donnelly and David Woolfson are named inventors on a patent application related to hydrogel-forming microneedle arrays (Donnelly, R.F., Woolfson A.D., McCarron, P.A., Morrow, D.I.J. Morrissey, A. (2007). Microneedles/delivery device and method. British patent application no. 0718996.2. Filed September 28, 2007. International publication no. WO2009040548. Approved for grant in Japan and China; USA, Europe, India and Australia pending). They are working with a number of companies with a view to commercialisation of this technology. They provide advice, through consultancy, to these companies. This does not alter our adherence to Drug Delivery & Translational Research policies on sharing data and materials. None of the other authors (Maelíosa T.C. McCrudden, Ahlam Zaid Alkilani, Aaron J. Courtenay, Cian M. McCrudden, Bronagh McCloskey, Christine Walker, Nida Alshraiedeh, Rebecca E.M. Lutton and Brendan F. Gilmore) has any competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCrudden, M.T.C., Alkilani, A.Z., Courtenay, A.J. et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv. and Transl. Res. 5, 3–14 (2015). https://doi.org/10.1007/s13346-014-0211-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-014-0211-1